
Concept explainers
(a)
Interpretation:
The most acidic proton, in the given species, is to be identified, and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the
Answer to Problem 6.50P
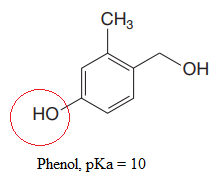
The most acidic proton in the given species along with its estimated pKa value is:
Explanation of Solution
The structure for the given compound is:
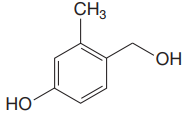
There are three protons that could be acidic. The proton attached to the carbon in methyl group, to the oxygen atom in alcohol, and the proton directly attached to the oxygen atom in phenol functional group are the protons that could be acidic.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having
The pKa value for the compound having
The pKa value for the compound having
Lower the pKa value, stronger is the acid, and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(b)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
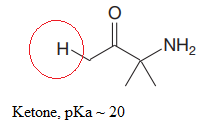
The most acidic proton in the given species along with its estimated pKa value is:
Explanation of Solution
The structure for the given compound is:
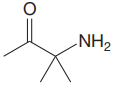
In the given structure, the proton attached to the nitrogen atom, and to the carbon atom next to the carbonyl group, could be the acidic protons.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having
The pKa value for the compound having
Lower the pKa value, stronger is the acid, and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(c)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
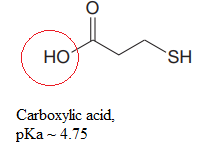
Explanation of Solution
The structure for the given compound is:
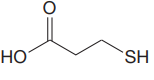
There are two protons that could be acidic. The proton attached to the oxygen atom which is directly bonded to the carbonyl group, and to the sulfur atom are the protons that could be acidic.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(d)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
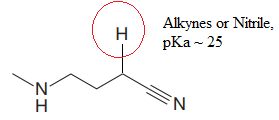
Explanation of Solution
The structure for the given compound is:
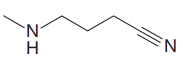
In the given structure, the proton attached to the nitrogen atom and to the triple bonded carbon atom could be the acidic protons.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(e)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
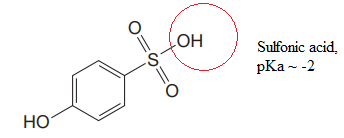
Explanation of Solution
The structure for the given compound is:
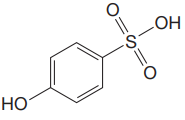
There are two protons that could be acidic. The proton attached to the carbon in methyl group, to the oxygen atom in alcohol, and the proton directly attached to the oxygen atom in phenol functional group are the protons that could be acidic.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(f)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
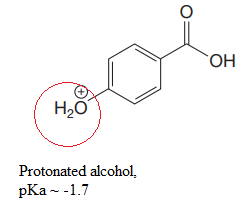
Explanation of Solution
The structure for the given compound is:
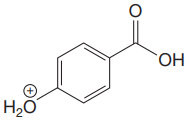
There are two protons that could be acidic. The proton attached to the carbon in methyl group, to the oxygen atom in alcohol, and the proton directly attached to the oxygen atom in phenol functional group are the protons that could be acidic.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having protonated
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated is the most acidic proton. The lowest pKa value is for protonated

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(g)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
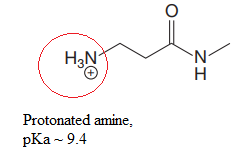
Explanation of Solution
The structure for the given compound is:
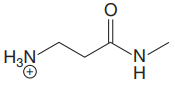
In the given structure, the proton attached to both nitrogen atoms could be acidic protons.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having protonated
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated with it is the most acidic proton. The lowest pKa value is for protonated

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
(h)
Interpretation:
The most acidic proton in the given species is to be identified and its pKa value is to be estimated.
Concept introduction:
An Acidic proton is the one which is directly bonded to an electronegative atom. The acidity of a compound is governed largely by the functional group on which the acidic proton is found. Nearby structural features such as highly electronegative substituent or presence of a double or triple bond can alter the acidity significantly. The pKa value for a particular compound is explained based on structural similarities of the compound and the compounds listed in Table 6-1.
Answer to Problem 6.50P
The most acidic proton in the given species along with its estimated pKa value is:
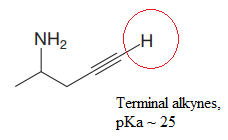
Explanation of Solution
The structure for the given compound is:
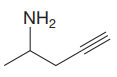
In the given structure, the protons attached to the terminal triple bonded carbon atom and to the nitrogen atom could be acidic protons.
According to Table 6-1, the relative pKa value of each of the protons is:
The pKa value for the compound having protonated
The pKa value for the compound having
Lower the pKa value, stronger is the acid and the proton associated with it is the most acidic proton. The lowest pKa value is for

The most acidic proton in the given structure is identified along with its estimated pKa value using Table 6-1.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- In the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forward
- draw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forward
- Post Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forwardIndicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forward
- How many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forwardIndicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


