
Concept explainers
(a)
Interpretation:
Whether reactants or products are favored at equilibrium and equilibrium constant value
Concept introduction:
For a reversible reaction, equilibrium constant (
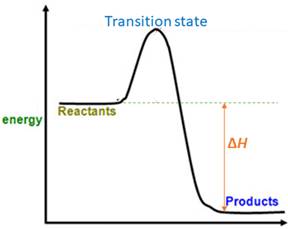
When energy is absorbed, the reaction is endothermic and the value of
Similarly, when energy is released, the reaction is exothermic and
Answer to Problem 48P
Reactants are favored.
Explanation of Solution
The magnitude of
Accordingly,
Therefore, reactants are favored at the equilibrium.
(b)
Interpretation:
Whether reactants or products are favored at equilibrium and change in enthalpy
Concept introduction:
For a reversible reaction, equilibrium constant (
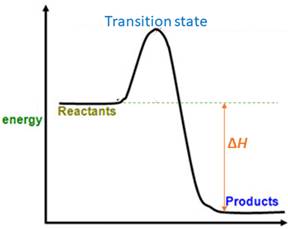
When energy is absorbed, the reaction is endothermic and the value of
Similarly, when energy is released, the reaction is exothermic and
Answer to Problem 48P
Reactants are favored.
Explanation of Solution
The value of
(c)
Interpretation:
Whether reactants or products are favored at equilibrium and equilibrium constant value 10,000 needs to be determined.
Concept introduction:
For a reversible reaction, equilibrium constant (
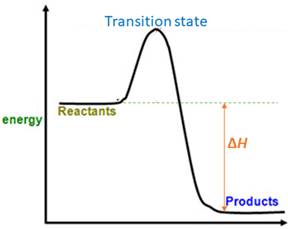
When energy is absorbed, the reaction is endothermic and the value of
Similarly, when energy is released, the reaction is exothermic and
Answer to Problem 48P
Products are favored.
Explanation of Solution
The magnitude of
Accordingly,
Therefore, products are favored at equilibrium.
(d)
Interpretation:
Whether reactants or products are favored at equilibrium and change in enthalpy
Concept introduction:
For a reversible reaction, equilibrium constant (
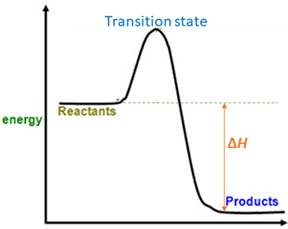
When energy is absorbed, the reaction is endothermic and the value of
Similarly, when energy is released, the reaction is exothermic and
Answer to Problem 48P
Products are favored.
Explanation of Solution
The value of
Want to see more full solutions like this?
Chapter 6 Solutions
General, Organic, and Biological Chemistry - 4th edition
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





