
Concept explainers
Determine the number of nodes along the internuclearaxis for each of the
Interpretation: The number of nodes along the internuclear axis for each of the
Concept introduction: Born-Oppenheimer approximation state that nuclei are heavier than electrons that considered fixed in space whereas the electrons move constantly around them. The Born−Oppenheimer approximation solves the electronic Schrodinger equation for
Answer to Problem 1P
The number of nodes in
Explanation of Solution
Node is point in molecular orbitals where probability to find the electron density is zero. Nodal plane is the imaginary plane where probability to find electrons is zero.
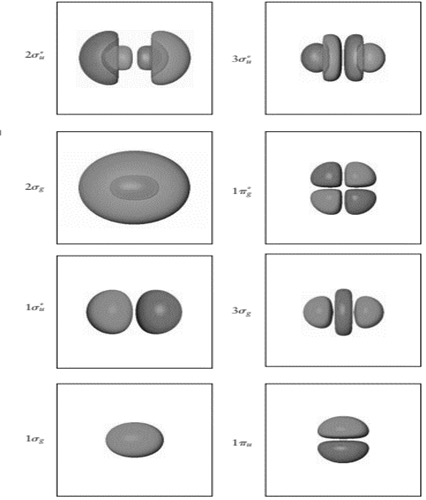
In the figure, node is the oval region. It is formed when plane white surface cut the spherical orbital into two equal halves having opposite symmetry. Also, there are six
In
In
In
In
In
In
Therefore, number of nodes in
Want to see more full solutions like this?
Chapter 6 Solutions
Principles of Modern Chemistry
- Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forwardCan you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!arrow_forward
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





