
(a)
Interpretation: Theacid base reaction of benzoic acid with sodium hydroxide needs to be explained.
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(b)
Interpretation: The acid, base, conjugated acid and conjugated base in the reaction of benzoic acid with sodium hydroxide needs to be determined.
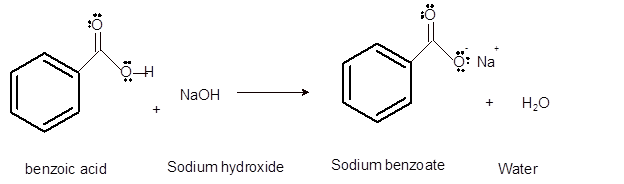
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
(c)
Interpretation: The solubility of benzoic acid and its conjugated base in diethyl ether and water needs to be explained.
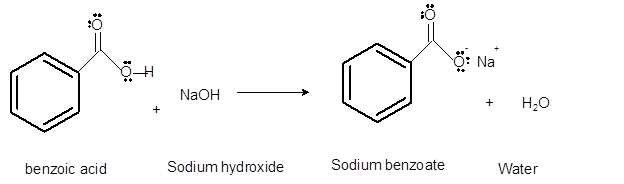
Concept Introduction:Bronsted and Lowry purposed the Bronsted-Lowry acid-base theory. It states that acid can give
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
- 8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forwardChoose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward
- 7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forwardWhat is the skeletal structure of the product of the following organic reaction?arrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardPlease help me answer the following questions using the data I included. 1&2arrow_forwardAssign all the Protons in HNMRarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

