
EBK BASIC CHEMISTRY
5th Edition
ISBN: 8220101472335
Author: Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5.1, Problem 5.8QAP
Interpretation Introduction
Interpretation:
To determine the given wavelength i.e. 850 nm in meters.
Concept introduction:
Wavelength: It may be defined as the distance between two successive crest of a wave. Unit of wavelength is ‘nm’.
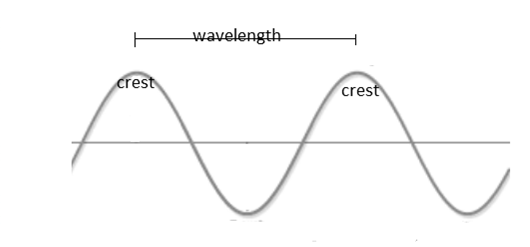
Generally, wavelength of radiations is very short. Therefore, it is usually measure in nanometres.
And,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the missing information
HO
NO2
Br2
FeBr3
to
CI
HO
H₂N
NO2
AICI3
Zn(Hg), HCI
1. NBS
2. t-BuONa
1. Br₂, FeBr3
2. CH3CI, AC13
3. Na2Cr2O7
Br
NH2
SO3H
HO
H₂N
Br
Assign the carbon numbered on CNMR
Find a structure formula for 108, 80, 78, 77, 51
Chapter 5 Solutions
EBK BASIC CHEMISTRY
Ch. 5.1 - What is meant by the wavelength of UV light?Ch. 5.1 - How are the wavelength and frequency of light...Ch. 5.1 - What is the difference between “white” light and...Ch. 5.1 - Prob. 5.4QAPCh. 5.1 - Prob. 5.5QAPCh. 5.1 - AM radio waves have a frequency of 8105s-1 ....Ch. 5.1 - Prob. 5.7QAPCh. 5.1 - Prob. 5.8QAPCh. 5.1 - Which ty pe of electromagnetic radiation, cell...Ch. 5.1 - Prob. 5.10QAP
Ch. 5.1 - Prob. 5.11QAPCh. 5.1 - Prob. 5.12QAPCh. 5.1 - Place the following types of electromagnetic...Ch. 5.1 - Prob. 5.14QAPCh. 5.2 - What feature of an atomic spectrum indicates that...Ch. 5.2 - Prob. 5.16QAPCh. 5.2 - Prob. 5.17QAPCh. 5.2 - Prob. 5.18QAPCh. 5.2 - Prob. 5.19QAPCh. 5.2 - Identify the photon in each pair with the greater...Ch. 5.3 - Describe the shape of each of the following...Ch. 5.3 - Prob. 5.22QAPCh. 5.3 - Prob. 5.23QAPCh. 5.3 - Prob. 5.24QAPCh. 5.3 - Prob. 5.25QAPCh. 5.3 - Prob. 5.26QAPCh. 5.3 - Prob. 5.27QAPCh. 5.3 - Prob. 5.28QAPCh. 5.4 - Compare the terms electron configuration and...Ch. 5.4 - Prob. 5.30QAPCh. 5.4 - 5.31 Draw the orbital diagram for each of the...Ch. 5.4 - 5.32 Draw the orbital diagram for each of the...Ch. 5.4 - Prob. 5.33QAPCh. 5.4 - Prob. 5.34QAPCh. 5.4 - Prob. 5.35QAPCh. 5.4 - Prob. 5.36QAPCh. 5.4 - Prob. 5.37QAPCh. 5.4 - Prob. 5.38QAPCh. 5.4 - Prob. 5.39QAPCh. 5.4 - Prob. 5.40QAPCh. 5.5 - Use the sublevel blocks on the periodic table to...Ch. 5.5 - Prob. 5.42QAPCh. 5.5 - Prob. 5.43QAPCh. 5.5 - Prob. 5.44QAPCh. 5.5 - Prob. 5.45QAPCh. 5.5 - Prob. 5.46QAPCh. 5.5 - Use the periodic table to give the symbol of the...Ch. 5.5 - Prob. 5.48QAPCh. 5.5 - Prob. 5.49QAPCh. 5.5 - Use the periodic table to give the number of...Ch. 5.6 - What do the group numbers from 1A (1) to 8A (18)...Ch. 5.6 - Prob. 5.52QAPCh. 5.6 - Write the group number using both A/B and 1 to 18...Ch. 5.6 - Prob. 5.54QAPCh. 5.6 - Prob. 5.55QAPCh. 5.6 - Prob. 5.56QAPCh. 5.6 - Prob. 5.57QAPCh. 5.6 - Indicate the number of valence electrons in each...Ch. 5.6 - Write the group number and draw the Lewis symbol...Ch. 5.6 - Prob. 5.60QAPCh. 5.6 - Prob. 5.61QAPCh. 5.6 - Prob. 5.62QAPCh. 5.6 - Prob. 5.63QAPCh. 5.6 - Prob. 5.64QAPCh. 5.6 - Prob. 5.65QAPCh. 5.6 - Prob. 5.66QAPCh. 5.6 - Prob. 5.67QAPCh. 5.6 - Prob. 5.68QAPCh. 5.6 - Prob. 5.69QAPCh. 5.6 - Prob. 5.70QAPCh. 5.6 - Prob. 5.71QAPCh. 5.6 - Fill in each of the following blanks using higher...Ch. 5.6 - Prob. 5.73QAPCh. 5.6 - Prob. 5.74QAPCh. 5.6 - Prob. 5.75QAPCh. 5.6 - Prob. 5.76QAPCh. 5 - Prob. 5.77FUCh. 5 - Prob. 5.78FUCh. 5 - Prob. 5.79UTCCh. 5 - Prob. 5.80UTCCh. 5 - Prob. 5.81UTCCh. 5 - Prob. 5.82UTCCh. 5 - Prob. 5.83UTCCh. 5 - Prob. 5.84UTCCh. 5 - Prob. 5.85UTCCh. 5 - Prob. 5.86UTCCh. 5 - Prob. 5.87AQAPCh. 5 - Prob. 5.88AQAPCh. 5 - Prob. 5.89AQAPCh. 5 - Prob. 5.90AQAPCh. 5 - Prob. 5.91AQAPCh. 5 - Prob. 5.92AQAPCh. 5 - 5.93 a. What electron sublevel starts to fill...Ch. 5 - Prob. 5.94AQAPCh. 5 - Prob. 5.95AQAPCh. 5 - Prob. 5.96AQAPCh. 5 - Prob. 5.97AQAPCh. 5 - Prob. 5.98AQAPCh. 5 - Prob. 5.99AQAPCh. 5 - Prob. 5.100AQAPCh. 5 - Prob. 5.101AQAPCh. 5 - Prob. 5.102AQAPCh. 5 - Prob. 5.103AQAPCh. 5 - Why is the ionization energy of Br lower than that...Ch. 5 - Prob. 5.105AQAPCh. 5 - Prob. 5.106AQAPCh. 5 - Prob. 5.107AQAPCh. 5 - Prob. 5.108AQAPCh. 5 - Prob. 5.109AQAPCh. 5 - Prob. 5.110AQAPCh. 5 - Prob. 5.111AQAPCh. 5 - Prob. 5.112AQAPCh. 5 - Prob. 5.113CQCh. 5 - Prob. 5.114CQCh. 5 - Prob. 5.115CQCh. 5 - Prob. 5.116CQCh. 5 - Prob. 5.117CQCh. 5 - Prob. 5.118CQCh. 5 - Prob. 5.119CQCh. 5 - Prob. 5.120CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PQ-10. What is the major product of this reaction? (A) (C) 930 Me HO O=S=O O-8-CF, C 어 Me H+ OH 270 O 0-5-0 O=S=O O-S-CF CF3 2arrow_forwardPredict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain.arrow_forwardQ2: Explain why epoxides that react in an SN1 manner will not show any stereochemical inversion in the product. Q3: Rationalize why Alcohol B will react under the indicated reaction conditions, but Alcohol A will not. A ☑ OH B OH PBr3 R-Brarrow_forward
- Q1: Predict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain. 1.) LDA, THF 2.) СОН CI OH H2SO4, heat OH m...... OH 1.) PCC, CH2Cl2 2.) CH3CH2MgBr, THF 3.) H3O+ 4.) TsCl, pyr 5.) tBuOK, tBuOH 1.) SOCI 2, CHCI 3 2.) CH3CH2ONA, DMF OH 1.) HBr 2.) Mg, THF 3.) H₂CO, THE 4.) H3O+ OH NaH, THFarrow_forwardWhat is the stepwise mechanism for this reaction?arrow_forwardDraw the major product of this reactionarrow_forward
- Please provide the IUPAC name for the compound shown herearrow_forwardProblem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY