
(a)
Interpretation:
The electron sublevel starts to fill after the completion of 3s sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
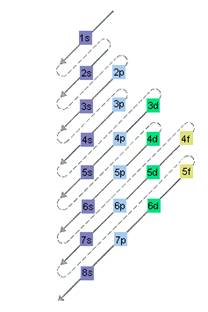
(b)
Interpretation:
The electron sublevel starts to fill after the completion of 4p sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
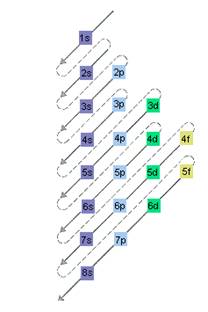
(c)
Interpretation:
The electron sublevel starts to fill after the completion of 3d sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
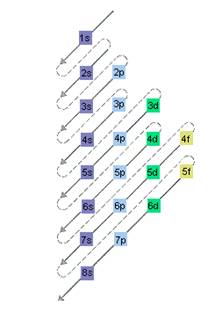
(d)
Interpretation:
The electron sublevel starts to fill after the completion of 3p sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
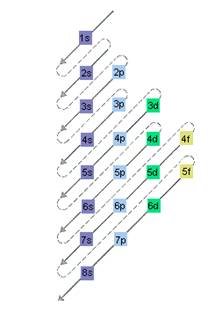
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK BASIC CHEMISTRY
- please solvearrow_forwardplease help with synthesisarrow_forward10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forward
- b) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forwardCH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forwardin the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forward
- help me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





