
Concept explainers
Problem Set
Enthalpy, Entropy, and Free Energy. The oxidation of glucose to carbon dioxide and water is represented by the following reaction, whether the oxidation occurs by combustion in the laboratory or by biological oxidation in living cells:
This reaction is highly exothermic, with an enthalpy change (ΔH) of –673 kcal/mol. As you know from Figure 5-9, ΔG for this reaction at 25°C is –686 kcal/mol, so the reaction is also highly exergonic.
(a) Explain in your own words what the ΔH and ΔG values mean. What do the negative signs mean in each case?
(b) What does it mean to say that the difference between the ΔG and ΔH values is due to entropy?
(c) Without doing any calculations, would ΔS for this reaction be positive or negative? Explain your answer.
(d) Now calculate ΔS for this reaction at 25°C. Does the calculated value agree in sign with your prediction in part c?
(e) What are the values of ΔG, ΔH, and ΔS for the reverse of the above reaction as carried out by a photosynthetic algal cell that is using CO2 and H2O to make C6H12O6?
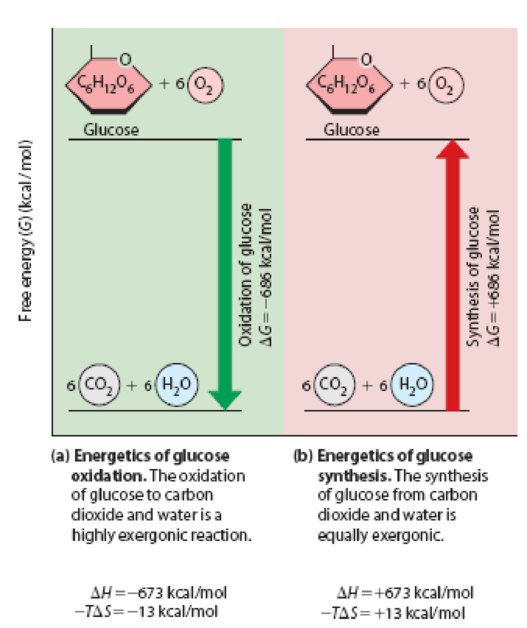
Figure 5-9 Changes in Free Energy for the Oxidation and Synthesis of Glucose. The exergonic oxidation of glucose shown in (a) has a large negative ΔG that is exactly equal in magnitude but opposite in sign to the large positive ΔG for the endergonic synthesis of glucose shown in (b).
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Becker's World of the Cell (9th Edition)
- 9 S es Read the section "Investigating Life: In (Extremely) Cold Blood." Then, drag and drop the terms on the left to complete the concept map. Red blood cells Genes Icefishes -have mutated have colorless Oxygen have few lack encode Blood Cellular respiration consists of- contain carries is a Platelets White blood cells carries low amounts of Hemoglobin is necessary for Plasma Protein Reset.arrow_forwardPlating 50 microliters of a sample diluted by a factor of 10-6 produced 91 colonies. What was the originalcell density (CFU/ml) in the sample?arrow_forwardEvery tutor here has got this wrong, don't copy off them.arrow_forward
- Suppose that the population from question #1 (data is in table below) is experiencing inbreeding depression (F=.25) (and no longer experiencing natural selection). Calculate the new expected genotype frequencies (f) in this population after one round of inbreeding. Please round to 3 decimal places. Genotype Adh Adh Number of Flies 595 Adh Adh 310 Adhs Adhs 95 Total 1000 fladh Adh- flAdn Adh fAdhs Adharrow_forwardWhich of the following best describes why it is difficult to develop antiviral drugs? Explain why. A. antiviral drugs are very difficult to develop andhave no side effects B. viruses are difficult to target because they usethe host cell’s enzymes and ribosomes tometabolize and replicate C. viruses are too small to be targeted by drugs D. viral infections usually clear up on their ownwith no problemsarrow_forwardThis question has 3 parts (A, B, & C), and is under the subject of Nutrition. Thank you!arrow_forward
- They got this question wrong the 2 previous times I uploaded it here, please make sure it's correvct this time.arrow_forwardThis question has multiple parts (A, B & C), and under the subject of Nutrition. Thank you!arrow_forwardCalculate the CFU/ml of a urine sample if 138 E. coli colonies were counted on a Nutrient Agar Plate when0.5 mls were plated on the NA plate from a 10-9 dilution tube. You must highlight and express your answerin scientific notatioarrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning




