
Concept explainers
Interpretation:
The procedure to make the specific concentration of solute in a solution have to describe.
Concept introduction:
The concentration of any solution is mean the amount of solute present in the specific volume of the solvent. Now the amount of solute present in the solution is expressed by different units like gram, gram-equivalent, gram-mole or mole etc. The amount of solvent to prepare the solution can be expressed in terms of weight or volume. The different unit of expression the concentration of a solution are- percentage strength, normality, molarity, molality, formality, gram per litter, mole fraction, parts per million etc.
Mass percent: In every 100 g solvent the amount of solute present in any solution is called the mass percent strength of the solution. Like- if in per 100 g of any solution 10 g pure 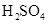 is present then the mass percent of the solute in the solvent is 10.
is present then the mass percent of the solute in the solvent is 10.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
- Draw the products formed when the following alkene is treated with 03 followed by Zn, H₂O. Click the "draw structure" button to launch the drawing utility. draw structure ...arrow_forwardCalculate the pH of 0.600 M solution of CH5N (Kb=4.37 x10-4) Hint: use assumption and check it!arrow_forwardDraw all stereoisomers formed when the following alkene is treated with mCPBA. Be sure to answer all parts. Part 1: How many stereoisomers of the product are possible? 1 Part 2 out of 2 Draw the product of the reaction, including stereochemistry. edit structure ...arrow_forward
- Draw the products formed when the following alkene is treated with 03 followed by Zn, H₂O. Be sure to answer all parts. draw structure ... smaller molar mass product draw structure ... larger molar mass productarrow_forwardComplete the two step reaction show the mechanism for all steps.arrow_forwardIdentify whether the reaction would proceed as a E1 or E2 mechanism.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





