
EBK CHEMISTRY FOR CHANGING TIMES
14th Edition
ISBN: 8220100663482
Author: MCCREARY
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 74AP
(a)
Interpretation Introduction
Interpretation:
% AE for reaction should be identified.
Concept introduction:
The combustion of pentane is follows:
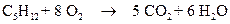
1 mole of pentane is producing 5 mol of carbon dioxide.
(b)
Interpretation Introduction
Interpretation:
Mass of CO2 produced should be identified.
Concept introduction:
The combustion of pentane is follows:
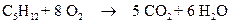
1 mole of pentane is producing 5 mol of carbon dioxide.
(c)
Interpretation Introduction
Interpretation:
Percentage yield for reaction should be identified.
Concept introduction:
The combustion of pentane is follows:
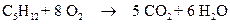
% yield = (Actual yield /theoretical yield) * 100
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check
the appropriate box.
Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below.
Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions
- just focus on the first stable product you expect to form in solution.
?
NH2
MgBr
Will the first product that forms in this reaction
create a new CC bond?
○ Yes
○ No
MgBr
?
Will the first product that forms in this reaction
create a new CC bond?
O Yes
O No
Click and drag to start drawing a
structure.
:☐
G
x
c
olo
Ar
HE
Predicting
As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule
with a new C - C bond as its major product:
H₂N
O
H
1.
?
2. H3O+
If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more
than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for
example to distinguish between major products with different stereochemistry.
0
If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank.
فا
Explanation
Check
Click and drag to start drawing a
structure.
Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers.
OH
OH
OH
OH
OH
OH
Chapter 5 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
Ch. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - What is Avogadro's hypothesis? How does it explain...Ch. 5 - Prob. 4RQCh. 5 - 5. Define or explain and illustrate the following...Ch. 5 - Prob. 6RQCh. 5 - Prob. 7PCh. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Ch. 5 - Consider the following equation. (a) Explain its...Ch. 5 - Prob. 12PCh. 5 - Prob. 13PCh. 5 - Prob. 14PCh. 5 - Prob. 15PCh. 5 - Prob. 16PCh. 5 - Prob. 17PCh. 5 - Prob. 18PCh. 5 - Prob. 19PCh. 5 - Prob. 20PCh. 5 - Prob. 21PCh. 5 - Prob. 22PCh. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - Prob. 25PCh. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Prob. 28PCh. 5 - Prob. 29PCh. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Consider the reaction for the combustion of octane...Ch. 5 - Prob. 37PCh. 5 - Toluene (C7H8) and nitric acid (HNO3) are used in...Ch. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Prob. 41PCh. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 -
44. What volume in liters of (a) 0.250 M NaOH...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - Prob. 50PCh. 5 - Prob. 51APCh. 5 - Prob. 52APCh. 5 - Prob. 53APCh. 5 - Prob. 54APCh. 5 - Prob. 55APCh. 5 - Prob. 56APCh. 5 - Prob. 57APCh. 5 - Prob. 58APCh. 5 - Prob. 59APCh. 5 - Prob. 60APCh. 5 - Prob. 61APCh. 5 - Prob. 62APCh. 5 - Prob. 63APCh. 5 - Prob. 64APCh. 5 - Prob. 65APCh. 5 - Prob. 66APCh. 5 - Prob. 67APCh. 5 - Evaluate this statement: ‘One cup of water has...Ch. 5 - Prob. 69APCh. 5 - Prob. 70APCh. 5 - Prob. 71APCh. 5 - Prob. 72APCh. 5 - Prob. 73APCh. 5 - Prob. 74APCh. 5 - Prob. 75APCh. 5 - Prob. 5.1CTECh. 5 - Prob. 5.2CTECh. 5 - Prob. 5.3CTECh. 5 - Prob. 5.4CTECh. 5 - Prob. 5.5CTECh. 5 - Prob. 1CGPCh. 5 - Prob. 2CGPCh. 5 - Prob. 1CHQCh. 5 - Prob. 2CHQCh. 5 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
- Given a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forwardTRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY