
EBK CHEMISTRY FOR CHANGING TIMES
14th Edition
ISBN: 8220100663482
Author: MCCREARY
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 35P
(a)
Interpretation Introduction
To determine: The moles of 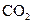 produced when
produced when 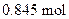 of butane is burned
of butane is burned
(b)
Interpretation Introduction
To determine: The moles of 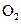 required when
required when 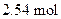 of butane is burned
of butane is burned
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
Chapter 5 Solutions
EBK CHEMISTRY FOR CHANGING TIMES
Ch. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - What is Avogadro's hypothesis? How does it explain...Ch. 5 - Prob. 4RQCh. 5 - 5. Define or explain and illustrate the following...Ch. 5 - Prob. 6RQCh. 5 - Prob. 7PCh. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Ch. 5 - Consider the following equation. (a) Explain its...Ch. 5 - Prob. 12PCh. 5 - Prob. 13PCh. 5 - Prob. 14PCh. 5 - Prob. 15PCh. 5 - Prob. 16PCh. 5 - Prob. 17PCh. 5 - Prob. 18PCh. 5 - Prob. 19PCh. 5 - Prob. 20PCh. 5 - Prob. 21PCh. 5 - Prob. 22PCh. 5 - Prob. 23PCh. 5 - Prob. 24PCh. 5 - Prob. 25PCh. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Prob. 28PCh. 5 - Prob. 29PCh. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Consider the reaction for the combustion of octane...Ch. 5 - Prob. 37PCh. 5 - Toluene (C7H8) and nitric acid (HNO3) are used in...Ch. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Prob. 41PCh. 5 - Prob. 42PCh. 5 - Prob. 43PCh. 5 -
44. What volume in liters of (a) 0.250 M NaOH...Ch. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 49PCh. 5 - Prob. 50PCh. 5 - Prob. 51APCh. 5 - Prob. 52APCh. 5 - Prob. 53APCh. 5 - Prob. 54APCh. 5 - Prob. 55APCh. 5 - Prob. 56APCh. 5 - Prob. 57APCh. 5 - Prob. 58APCh. 5 - Prob. 59APCh. 5 - Prob. 60APCh. 5 - Prob. 61APCh. 5 - Prob. 62APCh. 5 - Prob. 63APCh. 5 - Prob. 64APCh. 5 - Prob. 65APCh. 5 - Prob. 66APCh. 5 - Prob. 67APCh. 5 - Evaluate this statement: ‘One cup of water has...Ch. 5 - Prob. 69APCh. 5 - Prob. 70APCh. 5 - Prob. 71APCh. 5 - Prob. 72APCh. 5 - Prob. 73APCh. 5 - Prob. 74APCh. 5 - Prob. 75APCh. 5 - Prob. 5.1CTECh. 5 - Prob. 5.2CTECh. 5 - Prob. 5.3CTECh. 5 - Prob. 5.4CTECh. 5 - Prob. 5.5CTECh. 5 - Prob. 1CGPCh. 5 - Prob. 2CGPCh. 5 - Prob. 1CHQCh. 5 - Prob. 2CHQCh. 5 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
- For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forwardWrite the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.arrow_forwardName the following using IUPAC.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY