
Concept explainers
(a)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Mass number is the sum of the protons and neutrons in the nucleus.
For an element
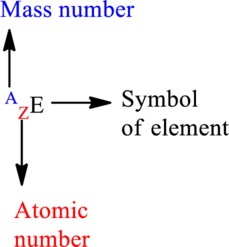
The letter A represents mass number.
The letter Z represents atomic number.
(a)
Answer to Problem 21PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(b)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 21PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(c)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 21PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
(d)
Interpretation:
The number of protons, neutrons and electrons in
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 21PE
The number of protons in
The number of electrons in
The number of neutrons in
Explanation of Solution
The atomic number of
The mass number of
Atomic number of an element tells about the number of protons present.
The number of protons present will be equal to the number of electrons.
Atomic number of
The number of neutrons is the difference between the mass number and the atomic number.
The number of neutrons is calculated as,
Number of neutrons=
Number of neutrons=
The number of protons in
The number of electrons in
The number of neutrons in
Want to see more full solutions like this?
Chapter 5 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





