
Concept explainers
Consider the following apparatus: a test tube coveredwith a nonpermeable elastic membrane inside a containerthat is closed with a cork. A syringe goes throughthe cork.
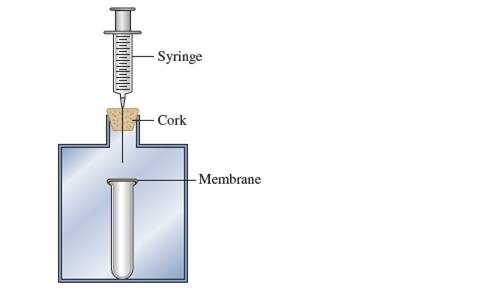
a. As you push down on the syringe, how does themembrane covering the test tube change?
b. You stop pushing the syringe but continue to hold itdown. In a few seconds, what happens to themembrane?
a)
Interpretation:Change in membrane cover if syringe is pushed down should be explained.
Concept introduction:Force per unit area is called pressure. Its SI unit is Pascal. Expression for pressure is as follows:
Explanation of Solution
When syringe is pushed down, pressure inside container and outside test tube increases. To balance effect of this increased pressure, elastic membrane expands in downward direction into test tube.
b)
Interpretation:Event that occurs if syringe is continued to be held down should be determined.
Concept introduction: Force per unit area is called pressure. Its SI unit is Pascal. Expression for pressure is as follows:
Explanation of Solution
When syringe is pushed down and hold down, constant pressure is applied on test tube and ultimately membrane will break.
Want to see more full solutions like this?
Chapter 5 Solutions
Chemical Principles
- One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forward
- Benzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forward
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





