
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.7, Problem 4.14P
Problem 4-14 Ethanol is produced industrially by the reaction of ethylene with water in the presence of an acid catalyst. How many grams of ethanol are produced from 7.24 mol of ethylene? Assume that excess water is present.
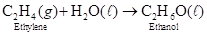
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For a titration of 20.00 mL of 0.0500 M H2SO4 with 0.100 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin; 2) 10.00 mL; 3) 20.00 mL; 4) 30.00 mL. Ka2 = 1.20×10-2 for H2SO4.
Write the systematic name of each organic molecule:
structure
name
show work. don't
give Ai generated
solution
Show work with explanation needed. Don't give Ai generated solution
Chapter 4 Solutions
Introduction to General, Organic and Biochemistry
Ch. 4.2 - Problem 4-1 Following is an unbalanced equation...Ch. 4.2 - Problem 4-2 Balance this equation:Ch. 4.2 - Prob. 4.3PCh. 4.3 - Problem 4-4 When a solution of copper(II)...Ch. 4.4 - Problem 4-5 In each equation, identify the...Ch. 4.5 - Problem 4-6 What is (a) the molecular weight of...Ch. 4.6 - Prob. 4.7PCh. 4.6 - Problem 4-8 We wish to weigh 2.84 mol of sodium...Ch. 4.6 - Problem 4-9 How many moles of C atoms, H atoms,...Ch. 4.6 - Problem 4-10 How many moles of copper(I) ions,...
Ch. 4.6 - Prob. 4.11PCh. 4.7 - Prob. 4.12PCh. 4.7 - Prob. 4.13PCh. 4.7 - Problem 4-14 Ethanol is produced industrially by...Ch. 4.7 - Prob. 4.15PCh. 4.7 - Prob. 4.16PCh. 4 - 4-17 Balance each equation.Ch. 4 - 4-18 Balance each equation.Ch. 4 - Prob. 4.19PCh. 4 - 4-20 Calcium oxide is prepared by heating...Ch. 4 - 4-21 The brilliant white light in some firework...Ch. 4 - Prob. 4.22PCh. 4 - 4-23 When solid carbon burns in a limited supply...Ch. 4 - Prob. 4.24PCh. 4 - 4-25 In the chemical test for arsenic, the gas...Ch. 4 - Prob. 4.26PCh. 4 - Prob. 4.27PCh. 4 - 4-28 Answer true or false. (a) A net ionic...Ch. 4 - 4-29 Balance these net ionic equations. (a)...Ch. 4 - 4-30 In the equation (a) Identify the spectator...Ch. 4 - 4-31 Predict whether a precipitate will form when...Ch. 4 - 4-32 When a solution of ammonium chloride is added...Ch. 4 - 4-33 When a solution of hydrochloric acid, HCl, is...Ch. 4 - Prob. 4.34PCh. 4 - Prob. 4.35PCh. 4 - 4-36 Using the solubility generalizations given in...Ch. 4 - 4-37 Answer true or false. (a) When a substance is...Ch. 4 - Prob. 4.38PCh. 4 - Prob. 4.39PCh. 4 - Prob. 4.40PCh. 4 - Prob. 4.41PCh. 4 - 4-42 Calculate the formula weight of: (a) KCl (b)...Ch. 4 - 4-43 Calculate the molecular weight of: (a)...Ch. 4 - 4-44 Answer true or false. (a) The mole is a...Ch. 4 - 4-45 Calculate the number of moles in: (a) 32 g of...Ch. 4 - 4-46 Calculate the number of grams in: (a) 1.77...Ch. 4 - 4-47 Calculate the number of moles of: (a) O atoms...Ch. 4 - 4-48 Calculate the number of moles of: (a) S2-...Ch. 4 - 4-49 Calculate the number of: (a) nitrogen atoms...Ch. 4 - 4-50 How many molecules are in each of the...Ch. 4 - 4-51 What is the mass in grams of each number of...Ch. 4 - 4-52 The molecular weight of hemoglobin is about...Ch. 4 - 4-53 A typical deposit of cholesterol, C27H46O, in...Ch. 4 - 4-54 Answer true or false. (a) Stoichiometry is...Ch. 4 - 4-55 For the reaction: (a) How many moles of N2...Ch. 4 - 4-56 Magnesium reacts with sulfuric acid according...Ch. 4 - 4-57 Chloroform, CHCl3, is prepared industrially...Ch. 4 - 4-58 At one time, acetaldehyde was prepared...Ch. 4 - 4-59 Chlorine dioxide, ClO2, is used for bleaching...Ch. 4 - 4-60 Ethanol, C2H6O, is added to gasoline to...Ch. 4 - 4-61 In photosynthesis, green plants convert CO2...Ch. 4 - 4-62 Iron ore is converted to iron by heating it...Ch. 4 - Prob. 4.63PCh. 4 - 4-64 Aspirin is made by the reaction of salicylic...Ch. 4 - 4-65 Suppose the preparation of aspirin from...Ch. 4 - 4-66 Benzene reacts with bromine to produce...Ch. 4 - 4-67 Ethyl chloride is prepared by the reaction of...Ch. 4 - 4-68 Diethyl ether is made from ethanol according...Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - 4-71 Which of these reactions are exothermic, and...Ch. 4 - Prob. 4.72PCh. 4 - Prob. 4.73PCh. 4 - Prob. 4.74PCh. 4 - Prob. 4.75PCh. 4 - Prob. 4.76PCh. 4 - 4-77 To convert 1 mol of iron(III) oxide to its...Ch. 4 - 4-78 (Chemical Connections 4A) How does fluoride...Ch. 4 - Prob. 4.79PCh. 4 - Prob. 4.80PCh. 4 - 4-81 (Chemical Connections 4C) Balance the lithium...Ch. 4 - 4-82 When gaseous dinitrogen pentoxide, N2O5, is...Ch. 4 - Prob. 4.83PCh. 4 - Prob. 4.84PCh. 4 - Prob. 4.85PCh. 4 - 4-86 When an aqueous solution of Na3PO4 is added...Ch. 4 - Prob. 4.87PCh. 4 - 4-88 Chlorophyll, the compound responsible for the...Ch. 4 - 4-89 If 7.0 kg of is added to 11.0 kg of to form...Ch. 4 - 4-90 Lead(lI) nitrate and aluminum chloride react...Ch. 4 - 4-91 Assume that the average red blood cell has a...Ch. 4 - 4-92 Reaction of pentane, C5H12, with oxygen, O2,...Ch. 4 - 4-93 Ammonia is prepared industrially by the...Ch. 4 - 4-94 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is...Ch. 4 - Prob. 4.95PCh. 4 - Prob. 4.96PCh. 4 - Prob. 4.97PCh. 4 - Prob. 4.98PCh. 4 - Prob. 4.99PCh. 4 - Prob. 4.100PCh. 4 - Prob. 4.101PCh. 4 - 4-102 Aspartame, an artificial sweetener used as a...Ch. 4 - 4-103 Caffeine, a central nervous system...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A Elschboard Part of SpeechT-D Alt Leaming App app.aktiv.com Curved arrows are used to illustrate the flow of electrons. Using the provided resonance structures, draw the curved electron- pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. Include all lone pairs and formal charges in the structures. Problem 45 of 10 I Select to Add Arrows N Please selarrow_forwardSo I'm working on molecular geometry. Can you help me with this stuff here and create three circles: one that's 120, one that’s 180, and one that’s 109.5?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 164 of N Select to Add Arrows CHI CH 1 1 1 Parrow_forward
- using these can you help me , I guess convert them to lewis dit structures or full drawn out skeletal and I guess is that what would help me depict the bond angle.arrow_forwardShow reaction mechanism with explanation.don't give Ai generated solutionarrow_forwardPlease answer the questions and provide detailed explanations.arrow_forward
- Show reaction mechanism. Don't give Ai generated solutionarrow_forwardPlease answer the questions and provide detailed explanation. Please also include the Hydrogens that are on the molecule to show how many signals there are.arrow_forwardCapp aktiv.com Part of Speech Table for Assi x Aktiv Learning App K Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 232 of 10 10: Mg Select to Add Arrows Br O H :0 CI:O H Mg THE + dy Undo Reset Done Brarrow_forward
- Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forwardNeed help with witharrow_forwardPlease answer the questions and provide detailed explanations.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY