
Concept explainers
(a)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.
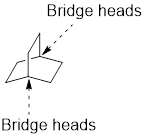
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(b)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] (each number represent number of carbon atoms) and placed in the middle of the parent.
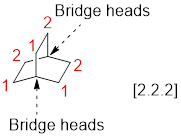
Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(c)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(d)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
(e)
Interpretation:
Given compound has to be named.
Concept Introduction:
Nomenclature of bicyclic compounds:
The organic compound naming is given by IUPAC (International Union for pure and applied chemistry). In the IUPAC names consist of certain rules for giving chemical names they are,
Identify and the parent: The term ‘Bicylo-’ is introduced in the name of the parent. Count the number of carbons excluding the bridge heads. In the compound below, each of the three paths has two carbons. These three numbers are ordered from largest to smallest, as [2.2.2] and placed in the middle of the parent.

Identify and name substituents: If substituent is present, the parent must be numbered properly in order to assign the locants to the substituent. To number the parent, start at one of the bridgeheads and begin numbering along the longest path, then go to the second longest path, and finally go along the shortest path.
Arrange the substituents alphabetically.
In the complex substituent in compounds, the substituent name is assigned by a name each of them based on numbers going away from the parent.
Trending nowThis is a popular solution!

Chapter 4 Solutions
ORGANIC CHEMISTRYPKGDRL+MLCRL MDL
- 4. Show the product(s) for the following reaction if it proceeds via the S42 mechanism AND if it proceeds via an Syt mechanism? Draw the mechanisms for both reactions and show all resonance structures for any intermediates. Would you expect the Su or Sy2 reaction to be favoured and why? NGOarrow_forwardI have a question here so in essence were just comparing the electronegativity values that are being given, soC and Cl, C and O, C and H to determine the partially positive, partially negative charges? So option I: Cl and C, option 2 O and C, option III C on its own correct?arrow_forwardDraw the less stable chair conformation of myo-inositol clearly indicating the axial and equatorial substituents as well as the cis and trans relationships of at least 3 OH groups. Draw a viable Newman Projection using any carbon carbon bond clearly showing a gauche interaction between the substituents.arrow_forward
- 5. What is the product for the following reaction for each step and draw the mechanism H 1. NaNH2 2, EtBrarrow_forwardmical lation or mula trations, AAAAAAAAAAAAA Experiment #8 Electrical conductivity & Electrolytes Conductivity of solutions FLINN Scientific conductivity meter scale - RED LED Scale 0 Green LED OFF OFF 1 Dim OFF 2 medium OFF Bright Dim 4 Very Bright Medium 3 LED Conductivity Low or None' Low Medium High very high SE = Strong Electrolyte, FE = Fair Electrolyte WE Weak Electrolyte, NE= Noni Electrolyte 9 0.1 M NaOH. 10. 0.1M NH3 11. D.1M HCT 12. 0.1 M HC2H3D2 13 0 m H2SO4 Prediction observed conductivity ? Very bright red, dim green (4) ? Saturated Bright red, dim green 3 Cacal) Bright red, dim green 3 Prediction Bright red, No green ? observed Bright red,dim green ? Conductivity Just red? I Can you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAAAA The light are not matching up with the scale So I'm confused about what I should be…arrow_forwardLabel these peaks in H- NMR and C- NMRarrow_forward
- Complete the following table. The only density needed is already given. Show your calculations in a neat and easy-to-follow manner in the space below the table. All units should be included and significant figures should be given close attention. Be sure to notice that the amount of material should be in millimoles rather than moles, and the theoretical mass of the product should in milligrams rather than grams. LOCH 3 + H2SO4 HNO 3 O=C-OCH 3 NO2 x H₂O F.W. 4.0 mL 1.3 M amount 0.50 mL in H2SO4 mg Theoretical Theoretical mmoles density 1.09arrow_forwardKumada Coupling: 1. m-Diisobutylbenzene below could hypothetically be synthesized by Friedel-Crafts reaction. Write out the reaction with a mechanism and give two reasons why you would NOT get the desired product. Draw the reaction (NOT a mechanism) for a Kumada coupling to produce the molecule above from m-dichlorobenzene. Calculate the theoretical yield for the reaction in question 2 using 1.5 g of p-dichlorobenzene and 3.0 mL isobutyl bromide. What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What other impurities are present in your product and how do you know?arrow_forwardWintergreen from Aspirin: 1. In isolating the salicylic acid, why is it important to press out as much of the water as possible? 2. Write the mechanism of the esterification reaction you did. 3. What characteristic absorption band changes would you expect in the IR spectrum on going from aspirin to salicyclic acid and then to methyl salicylate as you did in the experiment today? Give approximate wavenumbers associated with each functional group change. What signals appeared/disappeared/shifted that indicate that you have your intended product and not starting material? What other impurities are present in your product and how do you know?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





