
(a)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:
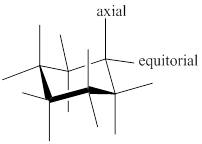
After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(b)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(c)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(d)
Interpretation:
Chair conformations of given compound should be drawn and most stable conformer is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

After ring flip of the conformer, axial position becomes equatorial and equatorial position becomes axial.
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRYPKGDRL+MLCRL MDL
- What are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardWhat is the reactant that makes the following product of the reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
- Draw the complete structural formula from each condensed structure. include all hydrogen atoms.arrow_forwardDraw the complete structural formula from each condensed structure. Include all hydrogen atoms.arrow_forwardIndicate how H2O2 intervenes in the synthesis of K4[Co2(C2O4)4(OH)2]. Write the reactions.arrow_forward
- Explain how, based on physical gas adsorption isotherms, we can determine whether multi-walled C nanotubes are open at their ends. Explain this.arrow_forwardcan somone answer pleasearrow_forwardConstruct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forward
- Indicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forwardConsider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





