
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 8PP
Practice Problem 4.8
Give names for the following substituted
(a)
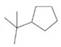
(b)
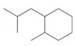
(c)
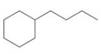
(d)
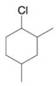
(e)
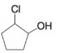
(f)
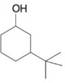
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Convert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).
Can you help me understand the CBC method on metal bridging by looking at this problem?
A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).
Chapter 4 Solutions
Organic Chemistry
Ch. 4 - Prob. 1PPCh. 4 - Which structure does not represent...Ch. 4 - Prob. 3PPCh. 4 - Draw bond-line formulas for all of the isomers of...Ch. 4 - Prob. 5PPCh. 4 - Draw bond-line formulas and give IUPAC...Ch. 4 - Draw bond-line formulas and give IUPAC...Ch. 4 - Practice Problem 4.8 Give names for the following...Ch. 4 - Prob. 9PPCh. 4 - Prob. 10PP
Ch. 4 - Prob. 11PPCh. 4 - Give the structures and IUPAC names for all the...Ch. 4 - Prob. 13PPCh. 4 - Practice Problem 4.14 Show by a calculation (using...Ch. 4 - Practice Problem 4.15 Write structures for the cis...Ch. 4 - Practice Problem 4.16
(a) Write structural...Ch. 4 - Practice Problem 4.17 Write a conformational...Ch. 4 - Practice Problem 4.18
(a) Write the two...Ch. 4 - Prob. 19PPCh. 4 - Practice Problem 4.20 (a) What is the index of...Ch. 4 - Practice Problem 4.21
Zingiberene, a fragrant...Ch. 4 - Practice Problem 4.22 Carbonyl groups also count...Ch. 4 - Prob. 23PCh. 4 - Give systematic IUPAC names for each of the...Ch. 4 - Prob. 25PCh. 4 - Write the structure and give the IUPAC systema.tic...Ch. 4 - 4.27. Write the structure(s) of the simplest...Ch. 4 - Prob. 28PCh. 4 - 4.29. Write structures for the following...Ch. 4 - Prob. 30PCh. 4 - A spiro ring junction is one where two rings that...Ch. 4 - 4.32. Tell what is meant by a homologous series...Ch. 4 - Four different cycloalkenes will all yield...Ch. 4 - 4.34. (a) Three different alkenes yield...Ch. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - 4.37. Write the structures of two chair...Ch. 4 - Prob. 38PCh. 4 - Without referring to tables, decide which member...Ch. 4 - Prob. 40PCh. 4 - 4.41. Which compound would you expect to be the...Ch. 4 - Prob. 42PCh. 4 - 4.43. Write the two chair conformations of each of...Ch. 4 - Provide an explanation for the surprising fact...Ch. 4 - Prob. 45PCh. 4 - 4.46. Specify the missing compounds and/or...Ch. 4 - Consider the cis and trans isomers of...Ch. 4 - Prob. 48PCh. 4 - Open the energy-minimized 3D Molecular Models on...Ch. 4 - 4.50. Open the 3D Molecular Models on the book’s...Ch. 4 - 4.51. Open the 3D Molecular Model on the book’s...Ch. 4 - 1. The predominant conformation for D-glucose is...Ch. 4 - Prob. 2LGPCh. 4 - When 1,2-dimethylcyclohexene is allowed to react...Ch. 4 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
Dr. Ara B. Dopsis and Dr. C. Ellie Gans are performing genetic crosses on daisy plants. They self-fertilize a b...
Genetic Analysis: An Integrated Approach (3rd Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
a. Which compound has the stretching vibration for its carbonyl group at the highest frequency: acetyl chloride...
Organic Chemistry (8th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License