
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 3LGP
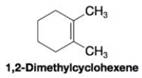
When 1,2-dimethylcyclohexene is allowed to react with hydrogen in the presence of a platinum catalyst, the product of the reaction is a cycloalkane that has a melting point of –50 °C and a boiling point of 130 °C (at 760 torr).
(a) What is the structure of the produce of this reaction?
(b) Consult an appropriate resource (such as the web or a CRC handbook) and tell which stereoisomer it is.
(c) What does this experiment suggest about the mode of addition of hydrogen to the double bond?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
In the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?
Chapter 4 Solutions
Organic Chemistry
Ch. 4 - Prob. 1PPCh. 4 - Which structure does not represent...Ch. 4 - Prob. 3PPCh. 4 - Draw bond-line formulas for all of the isomers of...Ch. 4 - Prob. 5PPCh. 4 - Draw bond-line formulas and give IUPAC...Ch. 4 - Draw bond-line formulas and give IUPAC...Ch. 4 - Practice Problem 4.8 Give names for the following...Ch. 4 - Prob. 9PPCh. 4 - Prob. 10PP
Ch. 4 - Prob. 11PPCh. 4 - Give the structures and IUPAC names for all the...Ch. 4 - Prob. 13PPCh. 4 - Practice Problem 4.14 Show by a calculation (using...Ch. 4 - Practice Problem 4.15 Write structures for the cis...Ch. 4 - Practice Problem 4.16
(a) Write structural...Ch. 4 - Practice Problem 4.17 Write a conformational...Ch. 4 - Practice Problem 4.18
(a) Write the two...Ch. 4 - Prob. 19PPCh. 4 - Practice Problem 4.20 (a) What is the index of...Ch. 4 - Practice Problem 4.21
Zingiberene, a fragrant...Ch. 4 - Practice Problem 4.22 Carbonyl groups also count...Ch. 4 - Prob. 23PCh. 4 - Give systematic IUPAC names for each of the...Ch. 4 - Prob. 25PCh. 4 - Write the structure and give the IUPAC systema.tic...Ch. 4 - 4.27. Write the structure(s) of the simplest...Ch. 4 - Prob. 28PCh. 4 - 4.29. Write structures for the following...Ch. 4 - Prob. 30PCh. 4 - A spiro ring junction is one where two rings that...Ch. 4 - 4.32. Tell what is meant by a homologous series...Ch. 4 - Four different cycloalkenes will all yield...Ch. 4 - 4.34. (a) Three different alkenes yield...Ch. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - 4.37. Write the structures of two chair...Ch. 4 - Prob. 38PCh. 4 - Without referring to tables, decide which member...Ch. 4 - Prob. 40PCh. 4 - 4.41. Which compound would you expect to be the...Ch. 4 - Prob. 42PCh. 4 - 4.43. Write the two chair conformations of each of...Ch. 4 - Provide an explanation for the surprising fact...Ch. 4 - Prob. 45PCh. 4 - 4.46. Specify the missing compounds and/or...Ch. 4 - Consider the cis and trans isomers of...Ch. 4 - Prob. 48PCh. 4 - Open the energy-minimized 3D Molecular Models on...Ch. 4 - 4.50. Open the 3D Molecular Models on the book’s...Ch. 4 - 4.51. Open the 3D Molecular Model on the book’s...Ch. 4 - 1. The predominant conformation for D-glucose is...Ch. 4 - Prob. 2LGPCh. 4 - When 1,2-dimethylcyclohexene is allowed to react...Ch. 4 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
22. How are stromal and parenchymal repair of a tissue different?
Principles of Anatomy and Physiology
25. The speed of sound in room temperature (20°C) air is 343 m/s; in room temperature helium, it is 1010 m/s. T...
College Physics: A Strategic Approach (3rd Edition)
68. Correct any incorrect equations. If no reaction occurs, write NO REACTION.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Ice cubes can disappear and food can dry out (freezer burn) in the freezer. That happens to the ice?
Fundamentals Of Thermodynamics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License