
Concept explainers
Interpretation:
The type of the reactions involved in change in the nucleus of an atom needs to be determined.
Concept introduction:
Nuclear reactions are main type of reactions which changes the nucleus from its original form. These reactions cause changes in the subatomic particles mainly the number of protons and the number of neutrons.
Answer to Problem 84A
There are mainly four types of reactions changing the nucleus of an atom.
- Nuclear fission.
- Nuclear fusion.
- Nuclear decay.
- Transmutation.
Explanation of Solution
- Nuclear fission − The first nuclear reaction is fission. The word fission explains the breakdown in the reaction. A heavy nucleus split or breakdown into two or more similar nuclei. These reactions are exothermic in nature.
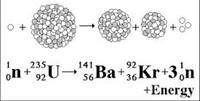
- Nuclear fusion − The second one reaction is the joining of nuclei of atoms. In this reaction, two simple or more atoms comes together and form a heavy nucleus. The fusion reaction of light elements can be highly exothermic in nature.
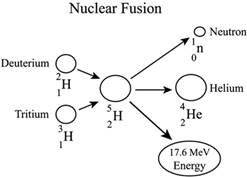
- Nuclear decay - The decay means that, in these reactions mainly the radiation occurs. There are three important radioactive reactions known as alpha, beta, and gamma decay. The number of protons or neutrons changes in these decays. Nuclear decay almost always involves large energy release in the form of radiation.
- Transmutation − This reaction is just reverse of the nuclear decay type reaction. These types of reactions are generally artificial process. They are non-spontaneous process. Transmutation involves increasing the mass of nuclei.
The examples are represented as follows:
a decay − 92U238→90Th234+ 2He4
ß decay − 53I131 →54Xe131 + -1e0
ϒdecay- 90Th234→90Th234 + ϒ
The nuclear reaction changes the stability as well as property of the product, these reactions are very helpful for the industrial level and for the research field but some reactions are harmful for the nature.
Chapter 4 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Anatomy & Physiology (2nd Edition)
Introductory Chemistry (6th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forwardPlease help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forward
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





