
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 68Q
Figure 5.8 shows energy differences for the combustion of H2, an exothermic
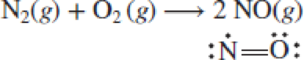
The bond energy for N═O is 630 kJ/mol. Sketch an energy diagram for this reaction and calculate the overall energy change.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic.
a)
HO
b)
Bri
H
HH
c)
d)
H
H H Br
0
None
Choose the option that is decreasing from biggest to smallest.
Group of answer choices:
100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm
10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m
10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m
100 m, 100 cm, 10000 mm, 100000 um, 10000000 nm
Chapter 4 Solutions
Chemistry in Context
Ch. 4.1 - Although power plants require several steps to...Ch. 4.1 - Prob. 4.3YTCh. 4.2 - Prob. 4.5CTCh. 4.3 - Prob. 4.7CTCh. 4.3 - Prob. 4.9YTCh. 4.4 - Prob. 4.10CTCh. 4.4 - The combustion of one gram of natural gas releases...Ch. 4.4 - Prob. 4.12CTCh. 4.5 - Prob. 4.14SCCh. 4.5 - Scientific Practices Coal Versus Ethanol On the...
Ch. 4.6 - Prob. 4.17YTCh. 4.7 - Beginning in the 1920s, the octane-booster...Ch. 4.9 - Prob. 4.21CTCh. 4.10 - Prob. 4.22CTCh. 4.11 - Have you ever been served cherries Jubilee or...Ch. 4.11 - Prob. 4.26CTCh. 4 - Prob. 4.1CTCh. 4 - Prob. 1QCh. 4 - Prob. 2QCh. 4 - Prob. 3QCh. 4 - Energy exists in different forms in our natural...Ch. 4 - A coal-burning power plant generates electrical...Ch. 4 - Prob. 6QCh. 4 - Prob. 7QCh. 4 - Prob. 8QCh. 4 - Mercury (Hg) is present in trace amounts in coal,...Ch. 4 - Prob. 10QCh. 4 - Here are the condensed structural formulas for two...Ch. 4 - Prob. 12QCh. 4 - Prob. 13QCh. 4 - Prob. 14QCh. 4 - During petroleum distillation, kerosene and...Ch. 4 - Prob. 16QCh. 4 - a. Write the balanced chemical equation for the...Ch. 4 - Prob. 18QCh. 4 - Prob. 19QCh. 4 - State whether these processes are endothermic or...Ch. 4 - Use the bond energies in Table 5.1 to calculate...Ch. 4 - Use the bond energies in Table 5.1 to calculate...Ch. 4 - Ethanol can be produced by fermentation. Another...Ch. 4 - Here are structural formulas for ethane, ethene...Ch. 4 - These three compounds all have the same chemical...Ch. 4 - Catalysts speed up cracking reactions in oil...Ch. 4 - Explain why cracking is a necessary part of the...Ch. 4 - Consider this equation representing the process of...Ch. 4 - Prob. 29QCh. 4 - Consider these three alcohols: methanol, ethanol,...Ch. 4 - Prob. 31QCh. 4 - Prob. 32QCh. 4 - Prob. 33QCh. 4 - Compare and contrast a molecule of biodiesel with...Ch. 4 - Use Figure 5.6 to compare the energy released for...Ch. 4 - Prob. 36QCh. 4 - The sustainability of burning coal (and other...Ch. 4 - In this chapter, we approximated the chemical...Ch. 4 - Prob. 39QCh. 4 - Compare the processes of combustion and...Ch. 4 - How might you explain the difference between...Ch. 4 - Write a response to this statement: Because of the...Ch. 4 - The concept of entropy and probability is used in...Ch. 4 - Bond energies such as those in Table 5.1 are...Ch. 4 - Use the bond energies in Table 5.1 to explain why...Ch. 4 - Prob. 46QCh. 4 - Prob. 47QCh. 4 - Prob. 48QCh. 4 - Prob. 49QCh. 4 - Prob. 50QCh. 4 - Prob. 51QCh. 4 - Prob. 52QCh. 4 - Prob. 53QCh. 4 - Use a diagram to show the relationship among these...Ch. 4 - On a timescale of a few years, the combustion of...Ch. 4 - Prob. 56QCh. 4 - Emissions of some pollutants are lower when...Ch. 4 - Although coal contains only trace amounts of...Ch. 4 - Prob. 59QCh. 4 - An article in Scientific American pointed out that...Ch. 4 - C. P. Snow, a noted scientist and author, wrote an...Ch. 4 - Chemical explosions are very exothermic reactions....Ch. 4 - Prob. 64QCh. 4 - Tetraethyllead (TEL) was first approved for use in...Ch. 4 - Tetraethyllead (TEL) has an octane rating of 270....Ch. 4 - Another type of catalyst used in the combustion of...Ch. 4 - Figure 5.8 shows energy differences for the...Ch. 4 - Prob. 69Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forwardNonearrow_forward
- Show work. don't give Ai generated solutionarrow_forwardPart II. count the expected number of signals in the 1H-NMR spectrum of these compounds HO 0 одев * Cl -cl "D"arrow_forwardPart I. Create a splitting tree diagram to predict the multiplet pattern of proton Hb in the compound below: 3 (Assume that "Jab >>> ³JbC) Ha Hb He он Ha NH2 Ha HCarrow_forward
- SH 0 iq noitzouDarrow_forwardNonearrow_forward+ HCl →? Draw the molecule on the canvas by choosing buttons from the Tools (for bonas), Atoms and Advanced Template toolbars. The single bond is active by default. + M C + H± 2D EXP. CONT. K ? L 1 H₁₂C [1] A HCN O S CH3 CH 3 CI Br HC H₂ CH CH CH3 - P Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY