
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 25Q
These three compounds all have the same chemical formula of C8H18. The hydrogen atoms and C─H bonds have been omitted for simplicity.
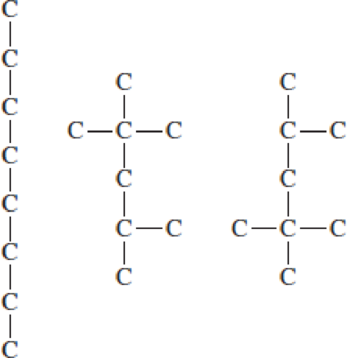
- a. For each compound, draw structural formulas that show the missing H atoms. All should have 18 H atoms.
- b. Which (if any) of these structural formulas are identical?
- c. Draw the structural formulas for any two additional isomers of C8H18.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the complete mechanism for this reaction:
.OH
مدید
OH
H2SO4
+ H₂O
To save you some time, the starting material has been copied into the first drawing area. However, you will still need to add any other reactants or catalysts that
take part in the reaction.
ན ི..
OH
Add/Remove step
Х
ด
ك
Click and drag to start
drawing a structure.
9:27 AM Tue Mar 4
←
Problem 64 of 15
#63%
Submit
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product
structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
0:0
0:0
:0:
N.
:0:
:O
:0:
H
H.
:0:
Select to Add Arrows
O
:0:
H
O
:0:
0:0.
S.
H
Select to Add Arrows
S
:0:
:0:
H
H
Order the following organic reactions by relative rate. That is, select '1' next to the reaction that will have the fastest initial rate, select '2' next to the reaction
that will have the next fastest initial rate, and so on. If two reactions will have very similar initial rates, you can select the same number next to both.
If a reaction will have zero or nearly zero initial rate, don't select a number and check the box in the table instead.
Note: the "Nu" in these reactions means "a generic nucleophile."
ملی
CI
:Nu
2
он
3
H
Reaction
Relative Rate
(Choose one) ▼
Nu
:CI:
zero or nearly zero
Nu
:Nu
bi
(Choose one)
zero or nearly zero
: Nu
لی
Nu
:H
(Choose one)
zero or nearly zero
Chapter 4 Solutions
Chemistry in Context
Ch. 4.1 - Although power plants require several steps to...Ch. 4.1 - Prob. 4.3YTCh. 4.2 - Prob. 4.5CTCh. 4.3 - Prob. 4.7CTCh. 4.3 - Prob. 4.9YTCh. 4.4 - Prob. 4.10CTCh. 4.4 - The combustion of one gram of natural gas releases...Ch. 4.4 - Prob. 4.12CTCh. 4.5 - Prob. 4.14SCCh. 4.5 - Scientific Practices Coal Versus Ethanol On the...
Ch. 4.6 - Prob. 4.17YTCh. 4.7 - Beginning in the 1920s, the octane-booster...Ch. 4.9 - Prob. 4.21CTCh. 4.10 - Prob. 4.22CTCh. 4.11 - Have you ever been served cherries Jubilee or...Ch. 4.11 - Prob. 4.26CTCh. 4 - Prob. 4.1CTCh. 4 - Prob. 1QCh. 4 - Prob. 2QCh. 4 - Prob. 3QCh. 4 - Energy exists in different forms in our natural...Ch. 4 - A coal-burning power plant generates electrical...Ch. 4 - Prob. 6QCh. 4 - Prob. 7QCh. 4 - Prob. 8QCh. 4 - Mercury (Hg) is present in trace amounts in coal,...Ch. 4 - Prob. 10QCh. 4 - Here are the condensed structural formulas for two...Ch. 4 - Prob. 12QCh. 4 - Prob. 13QCh. 4 - Prob. 14QCh. 4 - During petroleum distillation, kerosene and...Ch. 4 - Prob. 16QCh. 4 - a. Write the balanced chemical equation for the...Ch. 4 - Prob. 18QCh. 4 - Prob. 19QCh. 4 - State whether these processes are endothermic or...Ch. 4 - Use the bond energies in Table 5.1 to calculate...Ch. 4 - Use the bond energies in Table 5.1 to calculate...Ch. 4 - Ethanol can be produced by fermentation. Another...Ch. 4 - Here are structural formulas for ethane, ethene...Ch. 4 - These three compounds all have the same chemical...Ch. 4 - Catalysts speed up cracking reactions in oil...Ch. 4 - Explain why cracking is a necessary part of the...Ch. 4 - Consider this equation representing the process of...Ch. 4 - Prob. 29QCh. 4 - Consider these three alcohols: methanol, ethanol,...Ch. 4 - Prob. 31QCh. 4 - Prob. 32QCh. 4 - Prob. 33QCh. 4 - Compare and contrast a molecule of biodiesel with...Ch. 4 - Use Figure 5.6 to compare the energy released for...Ch. 4 - Prob. 36QCh. 4 - The sustainability of burning coal (and other...Ch. 4 - In this chapter, we approximated the chemical...Ch. 4 - Prob. 39QCh. 4 - Compare the processes of combustion and...Ch. 4 - How might you explain the difference between...Ch. 4 - Write a response to this statement: Because of the...Ch. 4 - The concept of entropy and probability is used in...Ch. 4 - Bond energies such as those in Table 5.1 are...Ch. 4 - Use the bond energies in Table 5.1 to explain why...Ch. 4 - Prob. 46QCh. 4 - Prob. 47QCh. 4 - Prob. 48QCh. 4 - Prob. 49QCh. 4 - Prob. 50QCh. 4 - Prob. 51QCh. 4 - Prob. 52QCh. 4 - Prob. 53QCh. 4 - Use a diagram to show the relationship among these...Ch. 4 - On a timescale of a few years, the combustion of...Ch. 4 - Prob. 56QCh. 4 - Emissions of some pollutants are lower when...Ch. 4 - Although coal contains only trace amounts of...Ch. 4 - Prob. 59QCh. 4 - An article in Scientific American pointed out that...Ch. 4 - C. P. Snow, a noted scientist and author, wrote an...Ch. 4 - Chemical explosions are very exothermic reactions....Ch. 4 - Prob. 64QCh. 4 - Tetraethyllead (TEL) was first approved for use in...Ch. 4 - Tetraethyllead (TEL) has an octane rating of 270....Ch. 4 - Another type of catalyst used in the combustion of...Ch. 4 - Figure 5.8 shows energy differences for the...Ch. 4 - Prob. 69Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9:12 AM Tue Mar 4 66% Problem 38 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the product formed in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Br2 FeBrз H (+) Br: H : Br----FeBr3 く a SU 00 nd earrow_forwardUnder aqueous acidic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: ☐ : P Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. CN H₂O H₂O H+ H+ Click and drag to start drawing a structure. Хarrow_forwardOrganic bases have lone pairs of electrons that are capable of accepting protons. Lone pair electrons in a neutral or negatively charged species, or pi electron pairs. Explain the latter case (pi electron pairs).arrow_forward
- Describe the propyl anion.arrow_forwardIndicate the names of these compounds (if they exist). 0: HỌC—NH CH3CH2-CH2arrow_forwardN Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forward
- The conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forward
- dont provide AI solution .... otherwise i will give you dislikearrow_forwardName these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License