
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 4, Problem 64P
Interpretation Introduction
Interpretation:
The reason should be explained for the given compound has four stereoisomers when it has only one asymmetric center.
Concept introduction:
The total number of stereoisomers can be calculated by using following formula,
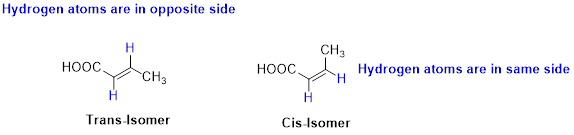
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of
Example:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
molecule
0=
OH
☐ ☐
type of molecule
(check all that apply)
fatty acid
monoglyceride
diglyceride
triglyceride
saturated
unsaturated
monounsaturated
☐ polyunsaturated
☐ ☐ ☐ ☐ ☐
010 0 0 0 0 0 0
☐ ☐ ☐ ☐☐☐☐
U
omega-3
omega-6
fatty acid
monoglyceride
diglyceride
triglyceride
saturated
unsaturated
monounsaturated
polyunsaturated
omega-3
omega-6
fatty acid
monoglyceride
diglyceride
triglyceride
saturated
unsaturated
monounsaturated
polyunsaturated
omega-3
omega-6
OH
OH
'☐
:
☑
ด
Suppose an alien life form has DNA just like human DNA
remain the same.)
-
except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients
Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled.
Note: be sure to draw the molecule as it would exist at physiological pH.
Click and drag to start drawing a
structure.
Predict the products of the following biochemical reaction:
CH2
CH-O
+ 3 KOH
CH2-0
In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the
No reaction box under the drawing area.
Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw
the complete right-hand side of the equation, including stoichiometric coefficients.
No reaction
Click and drag to start drawing a structure.
:
5
è
Chapter 4 Solutions
Essential Organic Chemistry, Global Edition
Ch. 4.1 - Draw the cis and trans isomers for the following:...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.1 - Prob. 6PCh. 4.2 - Prob. 7PCh. 4.2 - Tamoxifen slows the growth of some breast tumors...Ch. 4.2 - Draw and label the E and Z isomers for each of the...Ch. 4.2 - Prob. 10PCh. 4.2 - Name each of the following:Ch. 4.2 - Draw the structure of (Z)-2,3-dimethyl-3-heptene.
Ch. 4.3 - Prob. 13PCh. 4.4 - Prob. 14PCh. 4.5 - Prob. 16PCh. 4.6 - Prob. 17PCh. 4.7 - Assign relative priorities to the groups or atoms...Ch. 4.7 - Name the following:Ch. 4.7 - Prob. 22PCh. 4.7 - Draw a perspective formula for each of the...Ch. 4.8 - Prob. 24PCh. 4.8 - Prob. 27PCh. 4.9 - Prob. 28PCh. 4.9 - Prob. 29PCh. 4.9 - Prob. 30PCh. 4.10 - Prob. 31PCh. 4.10 - Prob. 32PCh. 4.10 - Prob. 33PCh. 4.11 - Prob. 34PCh. 4.11 - a. Draw the stereoisomers of...Ch. 4.11 - Prob. 37PCh. 4.11 - Prob. 38PCh. 4.12 - Which of the following compounds has a...Ch. 4.12 - Draw all the stereoisomers for each of the...Ch. 4.12 - Limonene exists as two different stereoisomers....Ch. 4 - a. Draw three constitutional isomers with...Ch. 4 - Which of the following have an asymmetric center?...Ch. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Of all the possible cyclooctanes that have one...Ch. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Draw the stereoisomers of 2,4-dichlorohexane....Ch. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Prob. 62PCh. 4 - Draw structures for each of the following: a....Ch. 4 - Prob. 64PCh. 4 - a. Draw all the isomers with molecular formula...Ch. 4 - Prob. 66PCh. 4 - Prob. 67PCh. 4 - Prob. 68PCh. 4 - Chloramphenicol is a broad-spectrum antibiotic...
Knowledge Booster
Similar questions
- Name 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forward
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
