
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 50P
Calculate the barrier to rotation for each bond highlighted in red.
a. 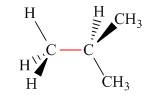 b.
b. 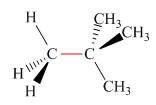
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw in a plane of symmetry for each molecule.
а.
b.
С.
d.
CI
Which labeled bond has the highest bond dissociation energy?
A.
B.
C.
D.
E.
Convert each three-dimensional model to a Newman projection around the indicated bond.
Chapter 4 Solutions
ORGANIC CHEMISTRY
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
22.102 Write the structures of the cis and tram isomers, if any, for the following compounds:
Chemistry: The Molecular Nature of Matter
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following species are conjugated? a. b. ن d. + نه +arrow_forwardThe sample that has no effect on the plane polarised light is a solution of (2S,3R)-diaminebutane (2S,3S)-diaminebutane (2R,3R)-diaminebutane An equimolar mixture of a & b a. b. C. d.arrow_forwardLabel each C–C double bond as E or Z.arrow_forward
- Answer the following questions about compounds A–D.a.How are the compounds in each pair related? Choose from constitutional isomers, stereoisomers, or identical molecules: A and B; A and C; B and D. b.Label each compound as a cis or trans isomer. c.Draw B as a hexagon with wedges and dashed wedges to show the stereochemistry of substituents. d.Draw a stereoisomer of A as a hexagon using wedges and dashed wedges to show the orientation of substituents.arrow_forwardHow many stereoisomers estuary for this molecule? Show how you know. A. 8 B. 3 C. 4 D. 6arrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Chemistry 5.66 A mixture contains equal amounts of compounds A-D. D B C A a. Which compounds alone are optically active? b. If the mixture was subjected to fractional distillation, how many fractions would be obtained? c. How many of these fractions would be optically active?arrow_forwardThe purine heterocycle occurs commonly in the structure of DNA. a. How is each N atom hybridized? b. In what type of orbital does each lone pair on a N atom reside? c. How many a electrons does purine contain? d. Why is purine aromatic? purinearrow_forwardLabel the following as aromatic, antiaromatic, or nonaromatic.arrow_forward
- 6. How many stereoisomers total are possible for each compound below appropriate line). a. b. Br Br Br place answer on thearrow_forwardWrite out the two-step sequence that converts benzene to each compound.arrow_forwardProblem 5.26 Without drawing out the structures, label each pair of compounds as enantiomers or diastereomers. a. (2R,3S)-hexane-2,3-diol and (2R,3R)-hexane-2,3-diol b. (2R,3R)-hexane-2,3-diol and (2S,3S)-hexane-2,3-diol c. (2R,3S,4R)-hexane-2,3,4-triol and (2S,3R,4R)-hexane-2,3,4-triolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License