
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 55P
For each compound drawn below:
a. Draw representations for the cis and trans isomers using a hexagon for the six-membered ring and wedges and dashed wedges for substituents.
b. Draw the two possible cahir conformations for the cis isomer. Which conformation, if either, is more stable?
c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable?
d. Which isomer, cis or trans, is more stable and why?
[1] 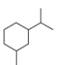 [2]
[2] 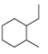 [3]
[3] 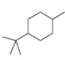
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1d. Use Le Chatelier's principle to describe the effect of the following changes on the
position of the Haber-Bosch equilibrium:
N2(g) + 3H2(g)= 2NH3(9) AH = -92kJ
Choose one of the following answers: shift to reactant side, shift to product side or no
change and draw the resulting graph.
I.
Increase the [N2(g)] Effect:
H₂
N₂
NH3
II.
Decrease the volume of the container. Effect:
H₂
N₂2
NH3
f) The unusual molecule [2.2.2] propellane is pictured.
1) Given the bond length and bond angles in the image, what hybridization scheme
best describes the carbons marked by the askerisks?
2) What types of orbitals are used in the bond between the two carbons marked by
the askerisks?
3) How does this bond compare to an ordinary carbon-carbon bond (which is usually
1.54 Å long)?
CH2 1.60Å
H₂C *
H₂C
CH2
C
H2C
*
C
Of
H₂
120°
e) Determine the hybridization and geometry around the indicated carbon atoms.
H3C
CH3
B
HC
CH2
A
C
C
C
CH3
Chapter 4 Solutions
ORGANIC CHEMISTRY
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
2. Which of the following is the best example of the use of a referent? _
a. A red bicycle
b. Big as a dump tru...
Physical Science
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 75.0 grams of an unknown metal was heated to 95.0°C, it was then placed into 150.0 grams of water at23.1°C, when the metal and water reached thermal equilibrium, the temperature was 27.8°C. Calculatethe specific heat of the metal. (Assume that the specific heat of water is 4.18 J/g °C)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardA 25.0 g sample of water was cooled from 23.9°C to 12.7°C, how much heat was released? (Assume thatthe specific heat of water is 4.18 J/g °C)arrow_forward
- Zeolites: environmental applications.arrow_forward" is The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 best described as a hybrid of several contributing resonance forms, two of which are shown here. HO :0: :Ö: HO + Bicarbonate is crucial for the control of body pH (for example, blood pH: 7.4). A more self-indulgent use is in baking soda, where it serves as a source of CO2 CO₂ 2 gas, which gives bread and pastry their fluffy constituency. (i) Draw at least one additional resonance form. = (ii) Using curved "electron-pushing" arrows, show how these Lewis structures may be interconverted by movement of electron pairs. (iii) Determine which form or forms will be the major contributor(s) to the real structure of bicarbonate, explaining your answer on the basis of the criteria in Section 1-5.arrow_forwardWhich of these is the best use of a volumetric flask? measuring how much liquid it contains delivering a precise amount of liquid to another container holding solutions making solutions of precise concentrationarrow_forward
- You're competing on a Great British television game show, and you need to bake a cake. The quantity for each ingredient is given in grams, but you haven't been given a kitchen scale. Which of these properties would correlate with the mass of a baking ingredient like eggs or milk? Check all that apply. depth of color viscosity volume densityarrow_forwardDraw a Lewis structure for each of the following species. Again, assign charges where appropriate. a. H-H¯ b. CH3-CH3 c. CH3+CH3 d. CH3 CH3 e. CH3NH3+CH3NH3 f. CH30-CH3O¯ g. CH2CH2 - h. HC2-(HCC) HC2 (HCC) i. H202×(HOOH) H₂O₂ (HOOH) Nortonarrow_forwardIs molecule 6 an enantiomer?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License