
Concept explainers
Using the cyclohexane with the C’s numbered as shown, draw a chair form that fits each
description.
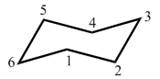
a. The ring has an axial
b. The ring has an equatorial
c. The ring has equatorial
Trending nowThis is a popular solution!

Chapter 4 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Chemistry & Chemical Reactivity
Chemistry
Chemistry: Structure and Properties
Organic Chemistry As a Second Language: Second Semester Topics
General Chemistry: Principles and Modern Applications (11th Edition)
- Using the cyclohexane with the C's numbered as shown, draw a chair form that fits each description. a.) The ring has an axial CH3 group at C1 and an equatorial OH on C2.b.) The ring has an equatorial CH3 group on C6 and an axial OH group on C4.c.) The ring has equatorial OH groups on C1, C2, and C5.arrow_forwardConsider 1,2-dimethylcyclohexane. a.Draw structures for the cis and trans isomers using a hexagon for the sixmembered ring. b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable? c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable? d.Which isomer, cis or trans, is more stable and why?arrow_forwardConsider 1,2-dimethylcyclohexane.a. Draw structures for the cis and trans isomers using a hexagon for the six-membered ring.b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable?c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable?d. Which isomer, cis or trans, is more stable and why?arrow_forward
- Draw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a.a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 group.arrow_forwardDraw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a. a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 grouparrow_forwardGlucose is a simple sugar with five substituents bonded to a sixmembered ring.a.Using a chair representation, draw the most stable arrangement of these substituents on the six-membered ring. b.Convert this representation to one that uses a hexagon with wedges and dashed wedges. c.Draw a constitutional isomer of glucose. d.Draw a stereoisomer that has an axial OH group on one carbon.arrow_forward
- narrow_forward1a. How many stereogenic centers are present 1c. Draw a three-dimensional structure of a in the structure below? Indicate them with asterisk(s). How many stereoisomers stereoisomers are possible? chiral compound with the molecular formula of C4H4Cl₂ that does not have a stereogenic carbon. In addition, draw the enantiomer of this compound. Number of stereogenic centers: Number of stereoisomers possible: 1b. Draw one of the two most stable stereoisomers of the compound in 1a using a planar structure with wedges and dashes. Now draw it in its preferred chair conformation. 1d. Draw two meso compounds with the molecular formula of C7H14.arrow_forwardGlucose is a simple sugar with five substituents bonded to a six-membered ring.a. Using a chair representation, draw the most stable arrangement of these substituents on the six-membered ring.b. Convert this representation into one that uses a hexagon with wedges and dashed wedges.c. Draw a constitutional isomer of glucose.d. Draw a stereoisomer that has an axial OH group on one carbon.arrow_forward
- For each compound drawn below: a. Draw representations for the cis and trans isomers using a hexagon for the six-membered ring, and wedges and dashes for substituents. b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable? c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable? d. Which isomer, cis or trans, is more stable and why? [1] [2] [3]arrow_forwardFor each compound drawn below: a. Draw representations for the cis and trans isomers using a hexagon for the six-membered ring, and wedges and dashed wedges for substituents. b.Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable? c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable? d.Which isomer, cis or trans, is more stable and why?arrow_forwardQuestion 2 pleasearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
