
Identify the atomic ground-state electron configurations that do not exist. For those that do exist, identify the element.
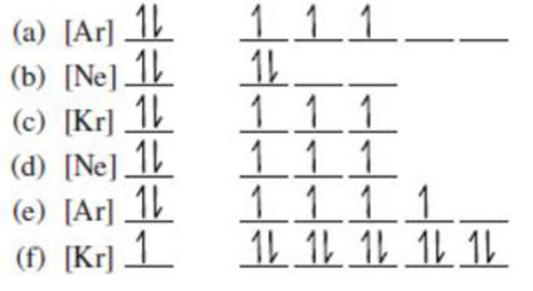
(a)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exist for (a) and the element is identified as vanadium.
Explanation of Solution
Ground-state electronic configuration of the given element (a) is,
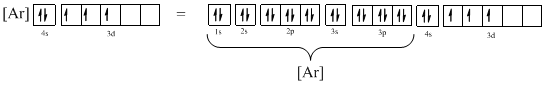
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and three electrons in 3d subshell. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this atomic ground-state configuration exists.
Element with the given atomic ground-state configuration is
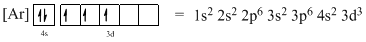
Argon is a noble gas and has a complete octet electronic configuration as
(b)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (b).
Explanation of Solution
Ground-state electronic configuration of (b)
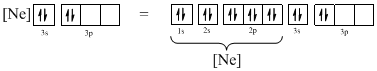
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete neon configuration along with two electrons in 3s subshell and two electrons in 3p subshell. This is derived as shown above. From the above drawn configuration we can find that the all the orbitals in 3p subshell is not singly filled before pairing. As this does not comply with Hund’s rule, the given atomic ground-state configuration does not exist.
(c)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (c).
Explanation of Solution
Ground-state electronic configuration of the given element (c)

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete krypton configuration along with two electrons in “s” subshell and three electrons in “p” subshell. This is derived as shown above. From the above drawn configuration we can find that the filling of orbitals does not take place according to the increasing order of energy. After filling of 5s orbital, 4d orbital has to be filled first before 5p orbital is getting filled. But in this case 5p subshell is filled with electrons. It does not comply with Hund’s rule and hence the given atomic ground-state configuration does not exist.
(d)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exists for (d) and the element is identified as phosphorous.
Explanation of Solution
Ground-state electronic configuration of the given element (d) is,

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and three electrons in 3d orbital. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this atomic ground-state configuration exists.
Element with the given atomic ground-state configuration

Neon is a noble gas and has a complete octet electronic configuration as
(e)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (e).
Explanation of Solution
Electronic configuration of given element (e) is,
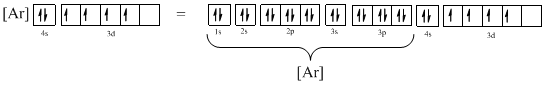
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and four electrons in 3d subshell. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this configuration exists.
Atomic ground-state configuration
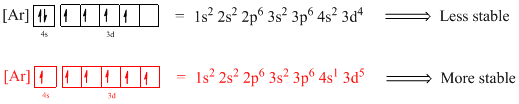
Argon is a noble gas and has a complete octet electronic configuration as
(f)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exists for (f) and the element is identified as silver.
Explanation of Solution
Ground-state electronic configuration of given element (f) is,

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete Krypton configuration along with one electrons in 5s orbital and ten electrons in 4d subshell. This is derived as shown above. As the filling of subshells follows the Hund’s rule, the given atomic ground-state configuration exist.
Element with the given atomic ground-state configuration
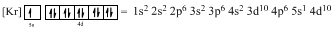
Argon is a noble gas and has a complete octet electronic configuration as
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: Atoms First
- 2. Explain why ice cubes formed from water of a glacier freeze at a higher temperature than ice cubes formed from water of an under- ground aquifer. Photodynamic/iStockphotoarrow_forwardShow reaction mechanism. don't give Ai generated solutionarrow_forward7. Draw the Lewis structures and molecular orbital diagrams for CO and NO. What are their bond orders? Are the molecular orbital diagrams similar to their Lewis structures? Explain. CO Lewis Structure NO Lewis Structure CO Bond Order NO Bond Order NO Molecular Orbital Diagram CO Molecular Orbital Diagramarrow_forward
- 5. The existence of compounds of the noble gases was once a great surprise and stimulated a great deal of theoretical work. Label the molecular orbital diagram for XeF (include atom chemical symbol, atomic orbitals, and molecular orbitals) and deduce its ground state electron configuration. Is XeF likely to have a shorter bond length than XeF+? Bond Order XeF XeF+arrow_forward6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+ B22+, B2, C22, B22 and N22+ Molecular Orbital Diagram B2 C22- B22- N22+ Which molecule is paramagnetic?arrow_forward3. Put the following species in order of increasing bond length by using molecular orbital diagrams and calculating their bond orders: F2, F2, F2+ Molecular Orbital Diagram F2 F2 F2+ Bond Order Shortest bond: Longest bondarrow_forward
- 3. Put the following species in order of increasing bond length by using molecular orbital diagrams and calculating their bond orders: F2, F2, F2+ Molecular Orbital Diagram F2 F2 F2+ Bond Orderarrow_forward4. The superoxide ion, Oz, plays an important role in the ageing processes that take place in organisms. Judge whether Oz is likely to have larger or smaller dissociation energy than 02. Molecular Orbital Diagram 02 02 Does O2 have larger or smaller dissociation energy?: Bond Orderarrow_forward1. How many molecular orbitals can be built from the valence shell orbitals in O2?arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





