
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
9th Edition
ISBN: 9781305968707
Author: Spencer L. Seager
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.57E
Use the periodic table and Table
a. 
b. 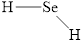
c. 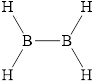
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The name of the following molecule is:
Ν
The table shows the tensile stress-strain values obtained for various
hypothetical metals. Based on this, indicate which is the most brittle
and which is the most tough (or most resistant).
Breaking strength Elastic modulus
Material Yield strength Tensile strength
Breaking strain
A
(MPa)
415
(MPa)
(MPa)
(GPa)
550
0.15
500
310
B
700
850
0.15
720
300
C
Non-effluence fracture
650
350
Please correct answer and don't used hand raiting
Chapter 4 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
Ch. 4 - Refer to the group numbers of the periodic table...Ch. 4 - Prob. 4.2ECh. 4 - Write abbreviated electronic configurations for...Ch. 4 - Write abbreviated electronic configurations for...Ch. 4 - Draw Lewis structure for the elements given in...Ch. 4 - Draw Lewis structures for the elements given in...Ch. 4 - Prob. 4.7ECh. 4 - Prob. 4.8ECh. 4 - Prob. 4.9ECh. 4 - Prob. 4.10E
Ch. 4 - Use the periodic table and predict the number of...Ch. 4 - Prob. 4.12ECh. 4 - Write a symbol for each of the following ions: a.A...Ch. 4 - Write a symbol for each of the following ions: a.A...Ch. 4 - Prob. 4.15ECh. 4 - Identify the element in period 3 that would form...Ch. 4 - Identify the noble gas that is isoelectronic with...Ch. 4 - Identify the noble gas that is isoelectronic with...Ch. 4 - Write equations to represent positive and negative...Ch. 4 - Prob. 4.20ECh. 4 - Write the formula for the ionic compound formed...Ch. 4 - Prob. 4.22ECh. 4 - Classify each of the following as a binary...Ch. 4 - Prob. 4.24ECh. 4 - Prob. 4.25ECh. 4 - Prob. 4.26ECh. 4 - Prob. 4.27ECh. 4 - Prob. 4.28ECh. 4 - Prob. 4.29ECh. 4 - Name the following binary ionic compounds: a. SrS...Ch. 4 - Name the following binary ionic compounds, using a...Ch. 4 - Name the following binary ionic compounds, using a...Ch. 4 - Prob. 4.33ECh. 4 - Prob. 4.34ECh. 4 - Prob. 4.35ECh. 4 - Write formulas for the following binary ionic...Ch. 4 - Prob. 4.37ECh. 4 - Prob. 4.38ECh. 4 - Identify the ions that would occupy lattice sites...Ch. 4 - Identify the ions that would occupy lattice sites...Ch. 4 - Calculate the mass in grams of positive ions and...Ch. 4 - Calculate the mass in grams of positive ions and...Ch. 4 - Prob. 4.43ECh. 4 - Prob. 4.44ECh. 4 - Represent the following reaction using Lewis...Ch. 4 - Prob. 4.46ECh. 4 - Prob. 4.47ECh. 4 - Represent the following molecules by Lewis...Ch. 4 - Draw Lewis structures for the following polyatomic...Ch. 4 - Prob. 4.50ECh. 4 - Prob. 4.51ECh. 4 - Prob. 4.52ECh. 4 - Predict the shape of each of the following...Ch. 4 - Prob. 4.54ECh. 4 - Prob. 4.55ECh. 4 - Prob. 4.56ECh. 4 - Use the periodic table and Table 4.4 to determine...Ch. 4 - Use Table 4.4 and classify the bonds in the...Ch. 4 - Use Table 4.4 and classify the bonds in the...Ch. 4 - Prob. 4.60ECh. 4 - On the basis of the charge distributions you drew...Ch. 4 - Prob. 4.62ECh. 4 - Prob. 4.63ECh. 4 - Prob. 4.64ECh. 4 - Show the charge distribution in the following...Ch. 4 - Prob. 4.66ECh. 4 - Prob. 4.67ECh. 4 - Prob. 4.68ECh. 4 - Prob. 4.69ECh. 4 - Prob. 4.70ECh. 4 - Prob. 4.71ECh. 4 - Prob. 4.72ECh. 4 - Prob. 4.73ECh. 4 - Prob. 4.74ECh. 4 - Prob. 4.75ECh. 4 - The covalent compounds ethyl alcohol and dimethyl...Ch. 4 - Prob. 4.77ECh. 4 - Prob. 4.78ECh. 4 - Prob. 4.79ECh. 4 - Prob. 4.80ECh. 4 - Prob. 4.81ECh. 4 - Prob. 4.82ECh. 4 - Suppose an element from group II(A)(2) and period...Ch. 4 - What would be the mass in grams of 0.200moles of...Ch. 4 - The ampere unit is used to describe the flow of...Ch. 4 - Prob. 4.86ECh. 4 - Prob. 4.87ECh. 4 - Prob. 4.88ECh. 4 - Prob. 4.89ECh. 4 - Prob. 4.90ECh. 4 - Prob. 4.91ECh. 4 - Prob. 4.92ECh. 4 - Prob. 4.93ECh. 4 - Prob. 4.94ECh. 4 - Prob. 4.95ECh. 4 - Noble gases: a.have low boiling points. b.are all...Ch. 4 - Prob. 4.97ECh. 4 - Name the type of bond that is formed when...Ch. 4 - Prob. 4.99ECh. 4 - A atom becomes an ion that possesses a negative...Ch. 4 - When calcium reacts with chlorine to form calcium...Ch. 4 - Prob. 4.102ECh. 4 - Prob. 4.103ECh. 4 - Which molecule below has a nonpolar bond in which...Ch. 4 - What is the correct formula for bismuth (III)...Ch. 4 - Which of the following species will combine with a...Ch. 4 - What type of bond is created when bromine and...Ch. 4 - The parts of an atom directly involved in ionic...Ch. 4 - In forming an ionic bond with an atom of chlorine,...Ch. 4 - In bonding, what would happen between the...Ch. 4 - Which compound contains a bond with no ionic...Ch. 4 - Prob. 4.112ECh. 4 - Which molecule is nonpolar and contains a nonpolar...Ch. 4 - Which of the following is a nonpolar covalent...Ch. 4 - Prob. 4.115ECh. 4 - Prob. 4.116E
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
60. The solar system is 25,000 light years from the center of our Milky Way galaxy. One light year is the dista...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The table shows the tensile stress-strain values obtained for various hypothetical metals. Based on this, indicate which material will be the most ductile and which the most brittle. Material Yield strength Tensile strength Breaking strain Breaking strength Elastic modulus (MPa) (MPa) (MPa) (GPa) A 310 340 0.23 265 210 B 100 120 0.40 105 150 с 415 550 0.15 500 310 D 700 850 0.14 720 210 E - Non-effluence fracture 650 350arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardDon't used hand raitingarrow_forward
- Consider the following Figure 2 and two atoms that are initially an infinite distance apart, x =00, at which point the potential energy of the system is U = 0. If they are brought together to x = x, the potential energy is related to the total force P by dU dx = P Given this, qualitatively sketch the variation of U with x. What happens at x=x? What is the significance of x = x, in terms of the potential energy? 0 P, Force 19 Attraction Total Repulsion x, Distance Figure 2. Variation with distance of the attractive, repulsive, and total forces between atoms. The slope dP/dx at the equilibrium spacing xe is proportional to the elastic modulus E; the stress σb, corresponding to the peak in total force, is the theoretical cohesive strength.arrow_forwardDenote the dipole for the indicated bonds in the following molecules. H3C ✓ CH3 B F-CCl 3 Br-Cl H3C Si(CH3)3 wwwwwww OH НО. HO HO OH vitamin C CH3arrow_forwardFor the SN2 reaction, draw the major organic product and select the correct (R) or (S) designation around the stereocenter carbon in the organic substrate and organic product. Include wedge-and-dash bonds and draw hydrogen on a stereocenter. Η 1 D EN Select Draw Templates More C H D N Erasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY