
Concept explainers
The police often use a device called a Breatkalyzer to test drivers suspected of being drunk. In one type of device, the breath of a driver suspected of driving under the influence of alcohol is bubbled through an orange solution containing potassium dichromate
(a) Classify each of the species in the Breathalyzer reaction as a strong electrolyte, weak electrolyte, or nonelectrolyte, (b) Write the ionic and net ionic equations for the Breathahzer reaction. (c) Determine the oxidation number of each element in the overall equation. (d) One manufacturer of Breathalyzers specifies a potassium dichromate concentration of 0.025 percent weight per volume
Interpretation:
The reactants and products in breathalyzer are to be classified as strong, weak, or non-electrolyte, the ionic and the net ionic equation for the reaction are to be represented, and the oxidation state of each element is to be determined in the reaction. The concentration of potassium dichromate in molarity is to be expressed and the volume of stock solution of
Concept introduction:
An electrolyte is a compound which dissociates into its corresponding ions when dissolved in water and conducts electricity. Electrolytes can be strong, weak, or non-electrolyte. The classification of the type of electrolyte is based on the formation of the ions when the electrolyte is dissolved in water.
An ionic reaction always follows the law of conservation of mass, according to which, when a chemical reaction occurs, the mass of ions in products should be equal to the mass of ions in reactants.
Oxidation number is the net charge on an element involved in the formation of a compound in a reaction. It is also known as oxidation state.
The concentration of a solution in terms of molarity is determined as follows:
Here,
Dilution is the process by which a less concentrated solution can be prepared from a more concentrated solution. But, the number of moles of solute remains the same in the original solution and the dilution. So, the concentration or volume of dilution can be determined as follows:
Here,
in ml,
Answer to Problem 153AP
Solution:
a)
b)
Ionic equation for breathalyzer is as follows:
The net ionic equation is as follows:
c)
Oxidation states of reactants and products are:
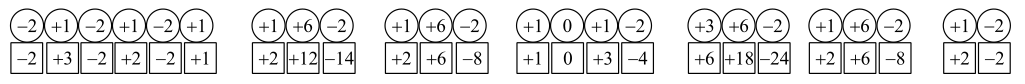
d)
The molar concentration of
e)
The volume of
f)
The molarity of
Explanation of Solution
a)
Given information: The reaction for breathalyzer is as follows:
a) Classify each of the species in the Breathalyzer reaction as a strong electrolyte, weak electrolite or nonelectrolyte.
b) The ionic and net ionic equations for the Breathalyzer reaction.
The ionic equation for the breathalyzer reaction is as follows:
The net ionic equation for the breathalyzer reaction is as follows:
c) The oxidation number of each element in the overall equation.
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
The oxidation numbers of elements in
d) The concentration in terms of molarity for the reaction
The concentration of
The molecular weight of
Substitute the values in the equation as follows:
e) The volume of
The concentration of stock solution is
Consider the two solutions, the stock solution is solution
Rearranging the equation to calculate the volume as follows:
f) The molarity of each ion in a
Potassium dichromate form the following ions on dissolution:
So, the concentration of these ions is as follows:
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry
Additional Science Textbook Solutions
Microbiology: Principles and Explorations
Cosmic Perspective Fundamentals
Biology: Life on Earth with Physiology (11th Edition)
Organic Chemistry
SEELEY'S ANATOMY+PHYSIOLOGY
General, Organic, and Biological Chemistry - 4th edition
- Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forward
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





