
a) Syndiotactic polyacrylonitrile
Interpretation:
Draw the three-dimensional structure of the polymeric products given in the question.
Concept introduction:
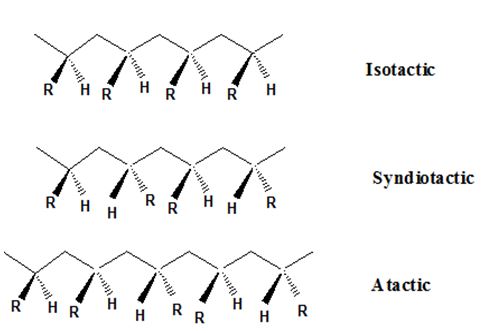
Isotactic, syndiotactic, and atactic are the stereochemical forms. The
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain growth
b) Atactic poly(methyl methacrylate)
Interpretation:
Draw the three-dimensional structure of the polymeric products given in the question.
Concept introduction:
Isotactic, syndiotactic, and atactic are the stereochemical forms. The polymer having the (-R) group on the same side of the macromolecule backbone (zig-zag bone) is known as isotactic polymer. An isotactic macromolecule consists of 100% meso diads.
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain growth polymerization mechanism must have chiral carbons to possess these structures. If the polymer formed does not have chiral carbon then it cannot occur in isotactic, syndiotactic and atactic forms.

c) Isotactic poly(vinyl chloride)
Interpretation:
Draw the three-dimensional structure of the polymeric products given in the question.
Concept introduction:
Isotactic, syndiotactic, and atactic are the stereochemical forms. The polymer having the (-R) group on the same side of the macromolecule backbone (zig-zag bone) is known as isotactic polymer. An isotactic macromolecule consists of 100% meso diads.
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain growth polymerization mechanism must have chiral carbons to possess these structures. If the polymer formed does not have chiral carbon then it cannot occur in isotactic, syndiotactic and atactic forms.

Want to see the full answer?
Check out a sample textbook solution
Chapter 31 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forward
- Draw a mental model for calcium chloride mixed with sodium phosphatearrow_forwardhere is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forward
- Drawing of 3-fluro-2methylphenolarrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





