
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305671874
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31.SE, Problem 16MP
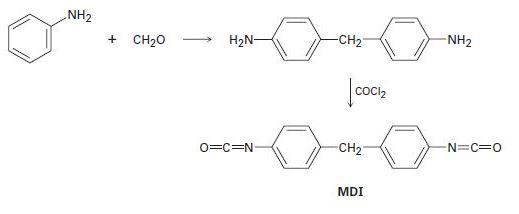
The polyurethane foam used for home insulation uses methane-diphenyldiisocyanate (MDI) as monomer. The MDI is prepared by acid-catalyzed reaction of aniline with formaldehyde, followed by treatment with phosgene, COCl2. Propose mechanisms for both steps.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Which pair of reagents will form the given product?
OH
X
+
Y
a.
CH3
b.
CH2CH3
༧་་
C. CH3-
CH2CH3
d.o6.(རི॰
e.
CH3
OCH2CH3
-MgBr
f. CH3-MgBr
g. CH3CH2-MgBr
-C-CH3
CH2CH3
Chapter 31 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
Ch. 31.1 - Order the following monomers with respect to their...Ch. 31.1 - Order the following monomers with respect to their...Ch. 31.1 - Prob. 3PCh. 31.2 - Prob. 4PCh. 31.2 - Prob. 5PCh. 31.3 - Prob. 6PCh. 31.3 - Prob. 7PCh. 31.4 - Prob. 8PCh. 31.4 - Show the mechanism of the nucleophilic addition...Ch. 31.5 - Prob. 10P
Ch. 31.6 - Prob. 11PCh. 31.6 - Prob. 12PCh. 31.SE - Prob. 13VCCh. 31.SE - Prob. 14VCCh. 31.SE - Prob. 15MPCh. 31.SE - The polyurethane foam used for home insulation...Ch. 31.SE - Prob. 17MPCh. 31.SE - Prob. 18MPCh. 31.SE - Prob. 19MPCh. 31.SE - Identify the monomer units from which each of the...Ch. 31.SE - Prob. 21APCh. 31.SE - Draw the structure of Kodel, a polyester prepared...Ch. 31.SE - Show the structure of the polymer that results...Ch. 31.SE - Prob. 24APCh. 31.SE - Prob. 25APCh. 31.SE - 1, 3-Cyclopentadiene undergoes thermal...Ch. 31.SE - Prob. 27APCh. 31.SE - Prob. 28APCh. 31.SE - Prob. 29APCh. 31.SE - Prob. 30APCh. 31.SE - Prob. 31APCh. 31.SE - Prob. 32APCh. 31.SE - Prob. 33APCh. 31.SE - Prob. 34APCh. 31.SE - Prob. 35AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY