
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 31.61P
Although chain branching in radical
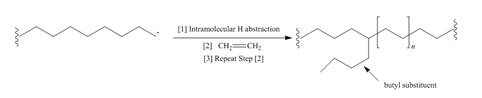
a. Draw a stepwise mechanism that illustrates which H must be intramolecularly abstracted to form butyl substituents.
b. Suggest a reason why the abstraction of this H is more facile than the abstraction of other H’s.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topic: Photochemistry and Photophysics of Supramolecules
Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 C
With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.
Chapter 31 Solutions
Organic Chemistry-Package(Custom)
Ch. 31 - Prob. 31.1PCh. 31 - Prob. 31.2PCh. 31 - Prob. 31.3PCh. 31 - Draw the mechanism for the radical polymerization...Ch. 31 - Prob. 31.5PCh. 31 - Prob. 31.6PCh. 31 - Prob. 31.7PCh. 31 - Prob. 31.8PCh. 31 - Prob. 31.9PCh. 31 - Prob. 31.10P
Ch. 31 - Prob. 31.11PCh. 31 - Problem 30.12
What polymer is formed by anionic...Ch. 31 - Prob. 31.13PCh. 31 - Prob. 31.14PCh. 31 - Problem 30.15
What polyamide is formed from each...Ch. 31 - Prob. 31.16PCh. 31 - Prob. 31.17PCh. 31 - Prob. 31.18PCh. 31 - Prob. 31.19PCh. 31 - Prob. 31.20PCh. 31 - Prob. 31.21PCh. 31 - Prob. 31.22PCh. 31 - Prob. 31.23PCh. 31 - Prob. 31.24PCh. 31 - Prob. 31.25PCh. 31 - Prob. 31.26PCh. 31 - 30.26 Draw the structure of the polymer formed by...Ch. 31 - Prob. 31.28PCh. 31 - Prob. 31.29PCh. 31 - Prob. 31.30PCh. 31 - Prob. 31.31PCh. 31 - Prob. 31.32PCh. 31 - Prob. 31.33PCh. 31 - Prob. 31.34PCh. 31 - Prob. 31.35PCh. 31 - Prob. 31.36PCh. 31 - Prob. 31.37PCh. 31 - Prob. 31.38PCh. 31 - Prob. 31.39PCh. 31 - 30.39 Draw a stepwise mechanism for the...Ch. 31 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 31 - Prob. 31.42PCh. 31 - Prob. 31.43PCh. 31 - 30.43 Although styrene undergoes both cationic and...Ch. 31 - 30.44 Rank the following compounds in order of...Ch. 31 - Prob. 31.46PCh. 31 - Prob. 31.47PCh. 31 - 30.47 Draw a stepwise mechanism for the following...Ch. 31 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 31 - Prob. 31.50PCh. 31 - Prob. 31.51PCh. 31 - Prob. 31.52PCh. 31 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 31 - Prob. 31.54PCh. 31 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 31 - Prob. 31.56PCh. 31 - Prob. 31.57PCh. 31 - 30.56 Compound A is a novel poly (ester amide)...Ch. 31 - Researchers at Rutgers University have developed...Ch. 31 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 31 - 30.59 Although chain branching in radical...Ch. 31 - Prob. 31.62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY