
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 31.53P
(a) Explain why poly (vinyl alcohol) cannot be prepared by the radical 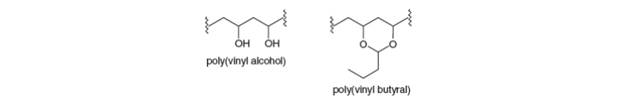
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In a photochemical reaction, how is the rate of the process related to its quantum yield?
Primary and global quantum yields in photochemistry. Define them and give their formulas. Differentiate between them.
Help me solve my HW
Chapter 31 Solutions
Organic Chemistry-Package(Custom)
Ch. 31 - Prob. 31.1PCh. 31 - Prob. 31.2PCh. 31 - Prob. 31.3PCh. 31 - Draw the mechanism for the radical polymerization...Ch. 31 - Prob. 31.5PCh. 31 - Prob. 31.6PCh. 31 - Prob. 31.7PCh. 31 - Prob. 31.8PCh. 31 - Prob. 31.9PCh. 31 - Prob. 31.10P
Ch. 31 - Prob. 31.11PCh. 31 - Problem 30.12
What polymer is formed by anionic...Ch. 31 - Prob. 31.13PCh. 31 - Prob. 31.14PCh. 31 - Problem 30.15
What polyamide is formed from each...Ch. 31 - Prob. 31.16PCh. 31 - Prob. 31.17PCh. 31 - Prob. 31.18PCh. 31 - Prob. 31.19PCh. 31 - Prob. 31.20PCh. 31 - Prob. 31.21PCh. 31 - Prob. 31.22PCh. 31 - Prob. 31.23PCh. 31 - Prob. 31.24PCh. 31 - Prob. 31.25PCh. 31 - Prob. 31.26PCh. 31 - 30.26 Draw the structure of the polymer formed by...Ch. 31 - Prob. 31.28PCh. 31 - Prob. 31.29PCh. 31 - Prob. 31.30PCh. 31 - Prob. 31.31PCh. 31 - Prob. 31.32PCh. 31 - Prob. 31.33PCh. 31 - Prob. 31.34PCh. 31 - Prob. 31.35PCh. 31 - Prob. 31.36PCh. 31 - Prob. 31.37PCh. 31 - Prob. 31.38PCh. 31 - Prob. 31.39PCh. 31 - 30.39 Draw a stepwise mechanism for the...Ch. 31 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 31 - Prob. 31.42PCh. 31 - Prob. 31.43PCh. 31 - 30.43 Although styrene undergoes both cationic and...Ch. 31 - 30.44 Rank the following compounds in order of...Ch. 31 - Prob. 31.46PCh. 31 - Prob. 31.47PCh. 31 - 30.47 Draw a stepwise mechanism for the following...Ch. 31 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 31 - Prob. 31.50PCh. 31 - Prob. 31.51PCh. 31 - Prob. 31.52PCh. 31 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 31 - Prob. 31.54PCh. 31 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 31 - Prob. 31.56PCh. 31 - Prob. 31.57PCh. 31 - 30.56 Compound A is a novel poly (ester amide)...Ch. 31 - Researchers at Rutgers University have developed...Ch. 31 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 31 - 30.59 Although chain branching in radical...Ch. 31 - Prob. 31.62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY