
Concept explainers
In a blast furnace at high temperature, iron(III) oxide in ore reacts with carbon monoxide to produce metallic iron and carbon dioxide. The liquid iron produced is cooled and weighed. The reaction is run repeatedly with the same initial mass of iron(III) oxide, 19.0 g, but differing initial masses of carbon monoxide. The masses of iron obtained arc shown in this graph.
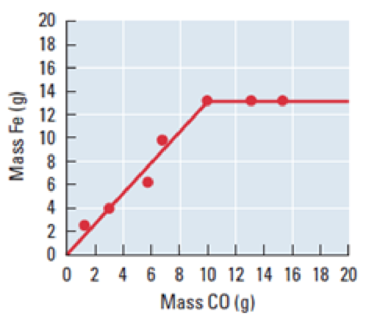
- (a) Write the balanced chemical equation for this reaction.
- (b) Calculate the mass of CO required to react completely with 19.0 g iron(III) oxide.
- (c) Calculate the mass of carbon dioxide produced when the reaction converts 10.0 g iron(III) oxide completely to products.
- (d) From the graph, determine which reactant is limiting when less than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (e) From the graph, determine which reactant is limiting when more than 10.0 g carbon monoxide is available to react with 19.0 g iron(III) oxide.
- (f) Calculate the percent yield if 24.0 g iron(III) oxide reacted with 20.0 g carbon monoxide to produce 15.9 g metallic iron.
- (g) Calculate the minimum mass of additional limiting reactant required to react with all of the excess of nonlimiting reactant from part (f).
(a)
Interpretation:
Iron
For the given reaction, balanced equation has to be written.
Concept introduction:
Balanced Chemical equation:
A balanced chemical equation is an equation which contains same elements in same number on both the sides (reactant and product side) of the chemical equation thereby obeying the law of conservation of mass.
Balancing the equation:
- There is a Law for conversion of mass in a chemical reaction i.e., the mass of total amount of the product should be equal to the total mass of the reactants.
- First write the skeletal reaction from the given information.
- Then count the number of atoms of each element in reactants as well as products.
- Place suitable coefficients in front of reactants as well as products until the number of atoms on each side (reactants and products) becomes equal.
Explanation of Solution
Given,
While balancing the equation, the subscripts cannot be altered but coefficients can be changed. There are two iron atoms in the left side and one iron atom in the right side of the reaction. Therefore, two iron atoms are added to right side of reaction. Now, the balanced equation is given below.
Three molecule of carbon monoxide is added to the left side of the reaction and three molecules of carbon dioxide is added to the right side of the reaction. Now, the balanced equation is given below.
(b)
Interpretation:
Mass of
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(c)
Interpretation:
Mass of carbon dioxide produced when the reaction converts
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Therefore, mass of
Mass of
(d)
Interpretation:
When less than
Concept introduction:
A limiting reactant is a reactant that is completely converted to products. Once all the limiting reactant is converted to products there is no other reactant to react.
Explanation of Solution
Given,
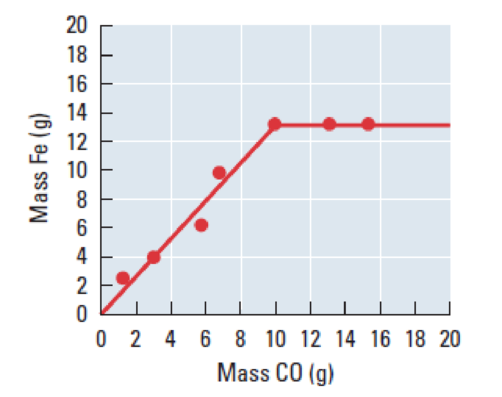
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The calculation, shows that
(e)
Interpretation:
When more than
Concept introduction:
Refer to part (d)
Explanation of Solution
Given,
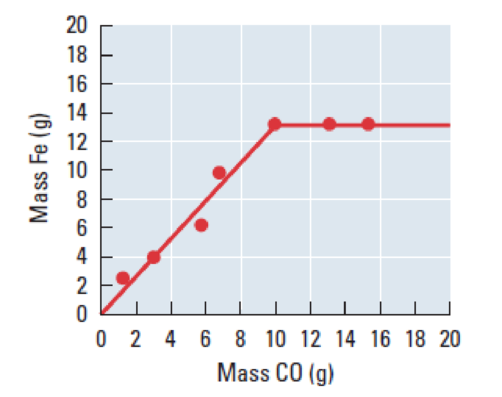
Figure 1
One mole of
The given graph between the mass of Fe and mass of carbon monoxide. The graph tells that the weight of the carbon monoxide is below
The graph tells that the weight of the carbon monoxide is more than
The calculation,
(f)
Interpretation:
When 24.0 g iron
Concept introduction:
The percent yield can be calculated by using following formula
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Amount of
The percent yield can be calculated by using following formula
The percent yield is
(g)
Interpretation:
The minimum mass of additional limiting reactant necessary to react with all of the excess of non-limiting reactant has to be calculated.
Explanation of Solution
Given,
The molar mass of
According to the balancing equation, one mole of
Given,
Number of moles of iron is calculated as follows,
Number of moles of iron is calculated as follows,
According to the mole calculation,
Gram of
Want to see more full solutions like this?
Chapter 3 Solutions
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
- ASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 IZ IN Molecule 4 Molecule 5 ZI none of the above ☐ Molecule 3 Х IN www Molecule 6 NH Garrow_forward
- Highlight each chiral center in the following molecule. If there are none, then check the box under the drawing area. There are no chiral centers. Cl Cl Highlightarrow_forwardA student proposes the following two-step synthesis of an ether from an alcohol A: 1. strong base A 2. R Is the student's proposed synthesis likely to work? If you said the proposed synthesis would work, enter the chemical formula or common abbreviation for an appropriate strong base to use in Step 1: If you said the synthesis would work, draw the structure of an alcohol A, and the structure of the additional reagent R needed in Step 2, in the drawing area below. If there's more than one reasonable choice for a good reaction yield, you can draw any of them. ☐ Click and drag to start drawing a structure. Yes No ロ→ロ 0|0 G Х D : ☐ பarrow_forwardटे Predict the major products of this organic reaction. Be sure to use wedge and dash bonds when necessary, for example to distinguish between different major products. ☐ ☐ : ☐ + NaOH HO 2 Click and drag to start drawing a structure.arrow_forward
- Shown below are five NMR spectra for five different C6H10O2 compounds. For each spectrum, draw the structure of the compound, and assign the spectrum by labeling H's in your structure (or in a second drawing of the structure) with the chemical shifts of the corresponding signals (which can be estimated to nearest 0.1 ppm). IR information is also provided. As a reminder, a peak near 1700 cm-1 is consistent with the presence of a carbonyl (C=O), and a peak near 3300 cm-1 is consistent with the presence of an O–H. Extra information: For C6H10O2 , there must be either 2 double bonds, or 1 triple bond, or two rings to account for the unsaturation. There is no two rings for this problem. A strong band was observed in the IR at 1717 cm-1arrow_forwardPredict the major products of the organic reaction below. : ☐ + Х ك OH 1. NaH 2. CH₂Br Click and drag to start drawing a structure.arrow_forwardNG NC 15Show all the steps you would use to synthesize the following products shown below using benzene and any organic reagent 4 carbons or less as your starting material in addition to any inorganic reagents that you have learned. NO 2 NC SO3H NO2 OHarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





