
Concept explainers
(a)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
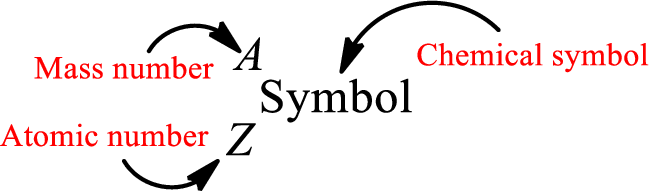
An element is a pure substance that cannot be broken by ordinary
(a)
Explanation of Solution
Given atom is
Atomic number is given as 50 and mass number is given as 118.
Therefore, the number of protons is 50, number of electrons is 50, and number of neutrons is 68.
The number of protons, electrons and neutrons present in the atom is given.
(b)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
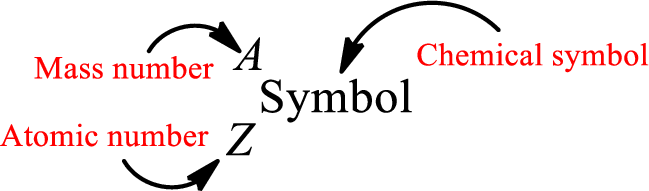
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(b)
Explanation of Solution
Given atom is
Atomic number is given as 78 and mass number is given as 195.
Therefore, the number of protons is 78, number of electrons is 78, and number of neutrons is 117.
The number of protons, electrons and neutrons present in the atom is given.
(c)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
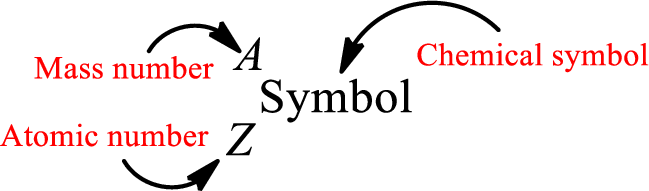
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(c)
Explanation of Solution
Given atom is
Atomic number is given as 31 and mass number is given as 72.
Therefore, the number of protons is 31, number of electrons is 31, and number of neutrons is 41.
The number of protons, electrons and neutrons present in the atom is given.
(d)
Interpretation:
The number of protons, neutrons and electrons present in the atom of
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
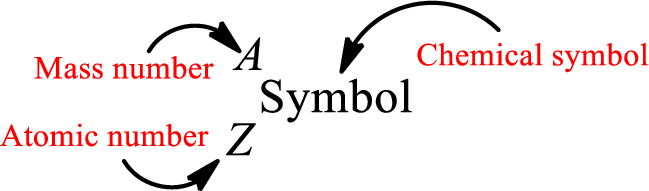
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(d)
Explanation of Solution
Given atom is
Atomic number is given as 12 and mass number is given as 26.
Therefore, the number of protons is 12, number of electrons is 12, and number of neutrons is 14.
The number of protons, electrons and neutrons present in the atom is given.
Want to see more full solutions like this?
Chapter 3 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward

 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





