
Identify each
represents a lactone-a cyclic ester- and which represents a lactam-a cyclic amide?
a. 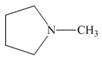 b.
b. 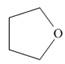 c.
c. 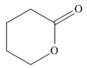 d.
d. 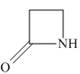
(a)
Interpretation: The functional group present in the given ring and the structure that represent a lactone and a lactam is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is amine. The given structure neither represents a lactone nor a lactam.
Explanation of Solution
The given structure is,
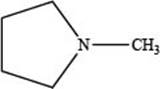
Figure 1
The given structure contains amine as a functional group. In the given structure, nitrogen is bonded to three carbon atoms. Therefore, the given ring is a tertiary amine.
Lactone is a cyclic ester, whereas lactam is a cyclic amide. Thus, the given structure neither represents a lactone nor a lactam.
The functional group present in the given ring is amine. The given structure neither represents a lactone nor a lactam.
(b)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is ether. The given structure neither represents a lactone nor a lactam.
Explanation of Solution
The given structure is,
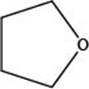
Figure 2
The given structure contains ether as a functional group. The given functional group is in cyclic form. Therefore, the given structure is cyclic ether. Lactone is a cyclic ester, whereas lactam is a cyclic amide. Thus, the given structure neither represents a lactone nor a lactam.
The functional group present in the given ring is ether. The given structure neither represents a lactone nor a lactam.
(c)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is ester. The given structure represents a lactone-cyclic ester.
Explanation of Solution
The given structure is,
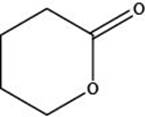
Figure 3
In the given structure, the functional group present is ester. The given functional group is in cyclic form. Therefore, the given structure represents a lactone-cyclic ester.
The functional group present in the given ring is ester. The given structure represents a lactone-cyclic ester.
(d)
Interpretation: The functional group present in the given ring and the structure that represent a lactone-cyclic ester and a lactam-a cyclic amide is to be stated.
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester |
Answer to Problem 3.33P
The functional group present in the given ring is amide. The given structure represents a lactam-a cyclic amide.
Explanation of Solution
The given structure is,
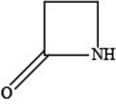
Figure 4
In the given structure, the functional group present is amide. The given functional group is in cyclic form. Therefore, the given structure represents a lactam-a cyclic amide.
The functional group present in the given ring is amide. The given structure represents a lactam-a cyclic amide.
Want to see more full solutions like this?
Chapter 3 Solutions
ORGANIC CHEMISTRY
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





