
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.57P
By using only electron density arguments, determine whether the following reactions will
occur.
a. 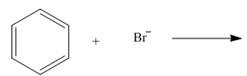 c.
c. 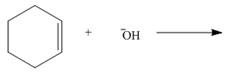
b. 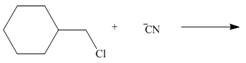 d.
d. 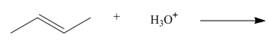
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If we have two compounds: acetone (CH3COCH3)
and acetic acid (CH3COOH); if we apply heat (A),
what product(s) are obtained?
QUESTION: Fill out the answers to the empty green boxes attached in the image.
*Ensure you all incorporate all 27 values (per column)*
You need to make a buffer by dissolving benzoic acid and sodium benzoate in
water. What is the mass of benzoic acid that you would weigh out, in mg, to
create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units
with the addition of 0.001 moles of acid or base?
Enter just the answer without the units (mg) - just the number will do!
Chapter 3 Solutions
ORGANIC CHEMISTRY
Ch. 3 - Prob. 3.1PCh. 3 - (a) Classify the carbon atoms in each compound as...Ch. 3 - Problem 3.3 Classify a carbon atom by the number...Ch. 3 - Classify each alkyl halide and alcohol as , or...Ch. 3 - Prob. 3.5PCh. 3 - Prob. 3.6PCh. 3 - Draw the structure of a compound of molecular...Ch. 3 - Prob. 3.8PCh. 3 - Prob. 3.9PCh. 3 - Draw the structure of a compound fitting each...
Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Which compound in each pair has the higher boiling...Ch. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.16PCh. 3 - Which compounds are water soluble? a. b. c.Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.19PCh. 3 - Prob. 3.20PCh. 3 - Prob. 3.21PCh. 3 - Prob. 3.22PCh. 3 - Problem 3.23 (a) What types of intermolecular...Ch. 3 - Prob. 3.24PCh. 3 - Prob. 3.25PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - Prob. 3.28PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.30PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.43PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 3.45PCh. 3 - 3.46 Rank the following compounds in order of...Ch. 3 - 3.47 Which of the following molecules can hydrogen...Ch. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.49PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - 3.56 Label the electrophilic and nucleophilic...Ch. 3 - 3.57 By using only electron density arguments,...Ch. 3 - 3.58 The composition of a cell membrane is not...Ch. 3 - Prob. 3.59PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 3.62PCh. 3 - Prob. 3.63PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the organic product: O A O B Ос ○ D -NH–CH3 + CH3 CH3 NEN C ? A CH3 CH3 NH- CH3 B CH3 CH3 N=N- C CH3 CH3 N=NNH CH3 D CH3 N=N CH3 NHCH3 LNH CHOarrow_forwardFinish the reaction- hand written pleasearrow_forwardGive the organic products: (benzyne) Br ? CH3 + K* :NH, liq NH3 HINT: Two products are formed. Each is a substituted aniline; they are isomers of each other. NH2 II I H₂N. CH3 CH3 III Select one: ○ A. I and II ○ B. I and III O C. I and IV O D. II and III O E. III and IV H₂N CH3 IV CH₂-NH2arrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Erase something Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terarrow_forwardQ14. Fill this chart: (please refer to ppt notes/browser to answer these questions) What alcohol is also called wood alcohol? What is the common name of ethanol? Draw the structure of phenol and thiophene? Are bigger chain alcohol like heptanol and octanol are soluble or insoluble in water and explain it ? Are ethers soluble or insoluble in water? What suffix and prefix are used for alcohol while naming alcohol and ether? What the process called when we add water to any alkene to make alcohol? Q16. Draw the diagram of following aromatic compound (practice from previous module) Aniline Phenol Benzoic acid Methyl benzoate Q17. a. Write the oxidation reactions for the 2 propanol. b. Write the oxidation reaction of the ethanol.arrow_forwardQuestion 11 of 18 (1 point) Question Attempt: 3 of How many signals do you expect in the 'H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. 1 For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box…arrow_forward
- Organic Chemistry Esterification reactions 1. Write the steps to prepare ester. 2. Write complete reaction of ethanol and acetic acid to make ester. 3. What does ester smell like? What are the uses of ester. 4. What the role of sulfuric acid in the esterification reactionarrow_forward11. Complete the following esterification reaction with names of all the reactants and products under. Hint: Remove the water and end up with ester R-C-OH + ROH R-C-OR + H₂O A carboxylic acid An alcohol An ester Water BYJU'S H-C-C O-H Нин C-C-C-H HAAA H O-C-C-C-H AAA Ethanoic acid Propanol Water Propyl ethanoate By com CH3COOH + CH3CH2CH2CH₂CH₂OH → Practice for alcohols aldehydes and ketones: 12. Draw the structures from the following names mixed of alcohol/aldehyde and ketone: a. 4-methyl cyclohexanone b. 3-methyl-2-pentenal c. 2,3-dimethylcyclohexanone d. 1,3propanediol or Propane 1,3 diol 13. Write systematic names for the following compounds identify functional group: a. b. (CH3)2CH-C OH c) CH(CH₂)-- OH -,-,arrow_forwardmay you please show all steps! i am having a hard time understanding and applying in this format, thank you!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY