
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.9, Problem 2.6CIAP
Interpretation Introduction
Interpretation:
Why the UV radiation from the sun are more damaging than the visible light has to be explained.
Concept Introduction:
The shorter the wavelength, the higher the energy, the longer the wavelength, the lower the energy.
Wave length region is shown in figure 1.
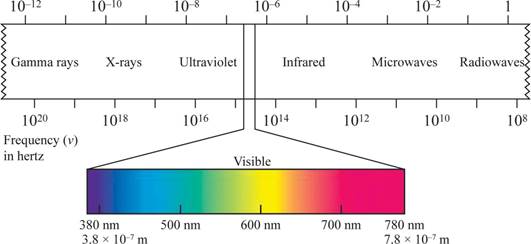
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Examine the metabolic pathway. The enzymes that catalyze each step are identified as "e" with a numeric subscript.
e₁
e3
e4
A
B
с
1°
B'
02
e5
e6
e7
E
F
Which enzymes catalyze irreversible reactions?
ப
e
ez
☐ ez
e4
☐
ப
es
26
5
e7
Which of the enzymes is likely to be the allosteric
enzyme that controls the synthesis of G?
€2
ез
e4
es
26
5
e7
An allosteric enzyme that follows the concerted model has an allosteric coefficient (T/R) of 300 in the absence of substrate.
Suppose that a mutation reversed the ratio.
Select the effects this mutation will have on the relationship between the rate of the reaction (V) and substrate
concentration, [S].
ㅁㅁㅁ
The enzyme would likely follow Michaelis-Menten kinetics.
The plot of V versus [S] would be sigmoidal.
The enzyme would mostly be in the T form.
The plot of V versus [S] would be hyperbolic.
The enzyme would be more active.
Penicillin is hydrolyzed and thereby rendered inactive by penicillinase (also
known as ẞ-lactamase), an enzyme present in some penicillin-resistant
bacteria. The mass of this enzyme in Staphylococcus aureus is 29.6 kDa.
The amount of penicillin hydrolyzed in 1 minute in a 10.0 mL. solution
containing 1.00 x 10 g of purified penicillinase was measured as a
function of the concentration of penicillin. Assume that the concentration of
penicillin does not change appreciably during the assay.
Plots of V versus [S] and 1/V versus 1/[S] for these data are shown.
Vo (* 10 M minute"¹)
7.0
6.0
5.0
4.0
3.0
20
1.0
0.0
о
10
20
30
1/Vo (* 10 M1 minute)
20
103
90
BO
70
50
[S] (* 100 M)
40
50
60
y=762x+1.46 × 10"
[Penicillin] (M)
Amount hydrolyzed (uM)
1
0.11
3
0.25
5
0.34
10
0.45
30
0.58
50
0.61
Chapter 2 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 2.1 - Prob. 2.1CIAPCh. 2.1 - For the Kanji character in the lower portion of...Ch. 2.2 - Use the list inside the front cover to identify...Ch. 2.3 - Prob. 2.2PCh. 2.3 - Prob. 2.3PCh. 2.3 - Prob. 2.4PCh. 2.4 - Prob. 2.5PCh. 2.4 - Prob. 2.6PCh. 2.4 - Prob. 2.7PCh. 2.4 - Prob. 2.8P
Ch. 2.4 - Prob. 2.9PCh. 2.5 - Prob. 2.10PCh. 2.5 - Prob. 2.11PCh. 2.5 - Prob. 2.12PCh. 2.5 - Prob. 2.13KCPCh. 2.5 - Prob. 2.3CIAPCh. 2.5 - Prob. 2.4CIAPCh. 2.6 - Prob. 2.14PCh. 2.7 - Prob. 2.15PCh. 2.7 - Write electron configurations for the following...Ch. 2.7 - Prob. 2.17PCh. 2.7 - Identify the atom with the following...Ch. 2.8 - Prob. 2.19PCh. 2.8 - Prob. 2.20PCh. 2.8 - Prob. 2.21PCh. 2.8 - Prob. 2.22KCPCh. 2.9 - Prob. 2.23PCh. 2.9 - Write electron-dot symbols for radon, lead, xenon,...Ch. 2.9 - Prob. 2.25PCh. 2.9 - Prob. 2.5CIAPCh. 2.9 - Prob. 2.6CIAPCh. 2 - Where on the following outline of a periodic table...Ch. 2 - Is the element marked in red on the following...Ch. 2 - Prob. 2.28UKCCh. 2 - What atom has the following orbital-filling...Ch. 2 - Use the following orbital-filling diagram to show...Ch. 2 - What four fundamental assumptions about atoms and...Ch. 2 - How do atoms of different elements differ?Ch. 2 - Prob. 2.33APCh. 2 - Prob. 2.34APCh. 2 - Prob. 2.35APCh. 2 - Prob. 2.36APCh. 2 - How many O atoms of mass 15.99 amu are in 15.99 g...Ch. 2 - Prob. 2.38APCh. 2 - What are the names of the three subatomic...Ch. 2 - Prob. 2.40APCh. 2 - Prob. 2.41APCh. 2 - Prob. 2.42APCh. 2 - Which of the following symbols represent isotopes...Ch. 2 - Prob. 2.44APCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - One of the most widely used isotopes in medical...Ch. 2 - Prob. 2.48APCh. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - For (a) rubidium (b) tungsten, (c) germanium, and...Ch. 2 - For (a) calcium, (b) palladium, (c) carbon, and...Ch. 2 - Prob. 2.58APCh. 2 - Prob. 2.59APCh. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Determine the number of unpaired electrons for...Ch. 2 - Without looking back in the text, write the...Ch. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Using n for the number of the valence shell and...Ch. 2 - What elements in addition to helium make up the...Ch. 2 - Prob. 2.81CPCh. 2 - What is the atomic number of the yet-undiscovered...Ch. 2 - Give the number of electrons in each shell for...Ch. 2 - Identify the highest-energy occupied subshell in...Ch. 2 - Prob. 2.85CPCh. 2 - Prob. 2.86CPCh. 2 - Germanium, atomic number 32, is used in building...Ch. 2 - Prob. 2.88CPCh. 2 - Prob. 2.89CPCh. 2 - What is wrong with the following electron...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.92CPCh. 2 - Prob. 2.93CPCh. 2 - Prob. 2.94CPCh. 2 - Prob. 2.95GPCh. 2 - Prob. 2.96GPCh. 2 - Prob. 2.97GPCh. 2 - Look again at the trends illustrated in Figures...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the four graphs shown. In each graph, the solid blue curve represents the unmodified allosteric enzyme and the dashed green curve represents the enzyme in the presence of the effector. Identify which graphs correctly illustrate the effect of a negative modifier (allosteric inhibitor) and a positive modifier (allosteric activator) on the velocity curve of an allosteric enzyme. Place the correct graph in the set of axes for each type of modifier. Negative modifier Reaction velocity - Positive modifier Substrate concentration - Reaction velocity →→→→ Substrate concentration Answer Bankarrow_forwardConsider the reaction: phosphoglucoisomerase Glucose 6-phosphate: glucose 1-phosphate After reactant and product were mixed and allowed to reach at 25 °C, the concentration of each compound at equilibrium was measured: [Glucose 1-phosphate] = 0.01 M [Glucose 6-phosphate] = 0.19 M Calculate Keq and AG°'. Код .0526 Incorrect Answer 7.30 AG°' kJ mol-1 Incorrect Answerarrow_forwardClassify each phrase as describing kinases, phosphatases, neither, or both. Kinases Phosphatases Neither Both Answer Bank transfer phosphoryl groups to acidic amino acids in eukaryotes may use ATP as a phosphoryl group donor remove phosphoryl groups from proteins catalyze reactions that are the reverse of dephosphorylation reactions regulate the activity of other proteins catalyze phosphorylation reactions PKA as an example turn off signaling pathways triggered by kinasesarrow_forward
- Consider the reaction. kp S P kg What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The rate constant for the reverse reaction (kr) increases. The reaction equilibrium is shifted toward the products. The concentration of the reactants is increased. The activation energy for the reaction is lowered. The formation of the transition state is promoted.arrow_forwardThe graph displays the activities of wild-type and several mutated forms of subtilisin on a logarithmic scale. The mutations are identified as: • The first letter is the one-letter abbreviation for the amino acid being altered. • The number identifies the position of the residue in the primary structure. ⚫ The second letter is the one-letter abbreviation for the amino acid replacing the original one. • Uncat. refers to the estimated rate for the uncatalyzed reaction. Log₁(S-1) Wild type S221A H64A -5 D32A S221A H64A D32A -10 Uncat. How would the activity of a reaction catalyzed by a version of subtilisin with all three residues in the catalytic triad mutated compare to the activity of the uncatalyzed reaction? It would have more activity, because the reaction catalyzed by the triple mutant is approximately three-fold faster than the uncatalyzed reaction. It would have less activity, because the reaction catalyzed by the triple mutant is approximately 1000-fold slower than the…arrow_forwardB Substrate Product AL Product Substrate Reaction progress- Reaction progress- omplete the passage describing the two reactions. In reaction A, the stability of the substrate is (AG) of the reaction is positive, Incorrect Answer greater than the stability of the product. The free-energy change Incorrect Answer so the reaction is considered In reaction B, the stability of the substrate is (AG) of the reaction is less than Incorrect Answer endergonic and Incorrect Answer not spontaneous. Incorrect Answer the stability of the product. The free-energy change negative, so the reaction is considered Incorrect Answer exergonic and spontaneous. Incorrect Answer Incorrect Answerarrow_forward
- Match the descriptions and compounds with the terms competitive, uncompetitive, and noncompetitive inhibition. Competitive inhibition Uncompetitive inhibition Noncompetitive inhibition Answer Bank inhibitor binds to the enzyme-substrate complex only Vmax remains the same but Ky increases inhibitor and substrate can bind simultaneously lowers Vmax and KM doxycycline sulfanilamide KM remains unchanged, but Vis lower prevents substrate from binding to the active site Rounduparrow_forwardDraw a pentapeptide made of the following amino acids: glycine, tyrosine, lysine, glutamate, and leucine at pH 1, pH 7, and pH 12. Draw the correct stereochemistry for each pentapeptide. Calculate the charge of the three compounds you've drawn and the PI.arrow_forward7. a. Complete the following redox reaction with the missing products NADH H b. Provide an arrow-pushing mechanism for the oxidation reaction. Use the explicit structure of NADH or NAD' as needed. For simplicity, use only the nicotinamide portion of the moleculearrow_forward
- Discuss the differences between eukaryotic and prokaryotic cells with regards to their genetic materials. Including in your discussion the structure and organisation of genetic material, as well as any implications these differences may have on cellular functions and evolution. Rubric Understanding of genetic material differences (provides a comprehensive and accurate explanation of the differences between eukaryotic and prokaryotic cells in terms of their genetic material, including the structure, organisation and function of genetic material in each cell type. Demonstrates a thorough understanding of the topic) Analysis of implications (analyze the differences between eukaryotic and prokaryotic cells in terms of their genetic material with depth and insight, discussing the implications of these differences on cellular functions and evolution. Provides specific examples and explanations.)arrow_forwardWhen was the dihydropyridine calcium channel blocker isradipine first patented and by whom? Please provide information on the origin and history of isradipine and who owns it/manufactures it.arrow_forward9) Below, there is a representation of an SDS-PAGE gel. Assuming the samples in the MW standard have masses of: 66 kDa, 45 kDa, 36 kDa, 29 kDa, 24 kDa, 20.1 kDa, and 14.2 kDa, a) Figure 4: indicate where each of the measurements were taken and label as in II.6. figure 2 above. b) As in II.7. Table 1 above create Table 2 using the data below. Determine the r.f. values for the MW standards, plot the relative mobility versus the log of the mass for the standards, and use the best fit straight line to determine the molecular weights of the proteins in the whey, peak 1, and peak 2 lanes. (5 pts—this will be scaled up appropriately if your gel did not develop properly) dye MW Whey Peak 1 Peak 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning
Comprehensive Medical Assisting: Administrative a...NursingISBN:9781305964792Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy CorreaPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Comprehensive Medical Assisting: Administrative a...
Nursing
ISBN:9781305964792
Author:Wilburta Q. Lindh, Carol D. Tamparo, Barbara M. Dahl, Julie Morris, Cindy Correa
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College



Phylogenetic Mysteries: Crash Course Zoology #12; Author: CrashCourse;https://www.youtube.com/watch?v=cVaw7nF72Aw;License: Standard youtube license