
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28.SE, Problem 24AP
The codon UAA stops protein synthesis. Why does the sequence UAA in the following stretch of mRNA not cause any problems?
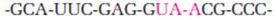
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5:45
Х
Select the final product for the following reaction sequence.
O
O
1. Mg. ether
2.D.O
Based on the chart
Two similarities between the molecule with alpha glycosidic linkages.
Two similarities between the molecules with beta glycosidtic linkages.
Two differences between the alpha and beta glycosidic linkages.
please help fill in the table
Chapter 28 Solutions
Organic Chemistry
Ch. 28.1 - Draw the full structure of the DNA dinucleotide...Ch. 28.1 - Draw the full structure of the RNA dinucleotide...Ch. 28.2 - What sequence of bases on one strand of DNA is...Ch. 28.4 - Show how uracil can form strong hydrogen bonds to...Ch. 28.4 - What RNA base sequence is complementary to the...Ch. 28.4 - From what DNA base sequence was the following RNA...Ch. 28.5 - List codon sequences for the following amino...Ch. 28.5 - List anticodon sequences on the tRNAs carrying the...Ch. 28.5 - What amino acid sequence is coded by the following...Ch. 28.5 - What is the base sequence in the original DNA...
Ch. 28.7 - Prob. 11PCh. 28.7 - Prob. 12PCh. 28.SE - Identify the following bases, and tell whether...Ch. 28.SE - Identify the following nucleotide, and tell how it...Ch. 28.SE - Amine bases in nucleic acids can react with...Ch. 28.SE - The final step in DNA synthesis is deprotection by...Ch. 28.SE - The final step in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of a...Ch. 28.SE - One of the steps in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of uridine...Ch. 28.SE - Human brain natriuretic peptide (BNP) is a small...Ch. 28.SE - Human and horse insulin both have two polypeptide...Ch. 28.SE - The DNA of sea urchins contains about 32% A. What...Ch. 28.SE - The codon UAA stops protein synthesis. Why does...Ch. 28.SE - Which of the following base sequences would most...Ch. 28.SE - For what amino acids do the following...Ch. 28.SE - Prob. 27APCh. 28.SE - Prob. 28APCh. 28.SE - Draw the complete structure of the ribonucleotide...Ch. 28.SE - Draw the complete structure of the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - Prob. 35APCh. 28.SE - Show the steps involved in a laboratory synthesis...Ch. 28.SE - Draw the structure of cyclic adenosine...Ch. 28.SE - Prob. 38AP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the structure of the DNA backbone?arrow_forward
- PLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forwardPlease use hand drawn structures when neededarrow_forward
- B. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forwardAnswe Answer A and B pleasearrow_forward
- 3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forwardLeucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY