
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28.SE, Problem 20MP
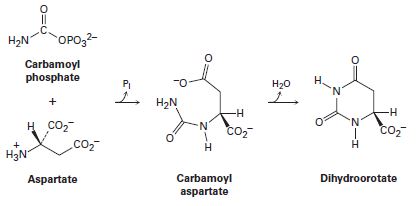
One of the steps in the biosynthesis of uridine monophosphate is the reaction of aspartate with carbamoyl phosphate to give carbamoyl aspartate followed by cyclization to form dihydroorotate. Propose mechanisms for both steps.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please provide with answer, steps and explanation of ideas to solve.
Define metal cluster and cage compound. Give some examples of both.
Please provide with answer, steps and explanation of ideas to solve.
Chapter 28 Solutions
Organic Chemistry
Ch. 28.1 - Draw the full structure of the DNA dinucleotide...Ch. 28.1 - Draw the full structure of the RNA dinucleotide...Ch. 28.2 - What sequence of bases on one strand of DNA is...Ch. 28.4 - Show how uracil can form strong hydrogen bonds to...Ch. 28.4 - What RNA base sequence is complementary to the...Ch. 28.4 - From what DNA base sequence was the following RNA...Ch. 28.5 - List codon sequences for the following amino...Ch. 28.5 - List anticodon sequences on the tRNAs carrying the...Ch. 28.5 - What amino acid sequence is coded by the following...Ch. 28.5 - What is the base sequence in the original DNA...
Ch. 28.7 - Prob. 11PCh. 28.7 - Prob. 12PCh. 28.SE - Identify the following bases, and tell whether...Ch. 28.SE - Identify the following nucleotide, and tell how it...Ch. 28.SE - Amine bases in nucleic acids can react with...Ch. 28.SE - The final step in DNA synthesis is deprotection by...Ch. 28.SE - The final step in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of a...Ch. 28.SE - One of the steps in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of uridine...Ch. 28.SE - Human brain natriuretic peptide (BNP) is a small...Ch. 28.SE - Human and horse insulin both have two polypeptide...Ch. 28.SE - The DNA of sea urchins contains about 32% A. What...Ch. 28.SE - The codon UAA stops protein synthesis. Why does...Ch. 28.SE - Which of the following base sequences would most...Ch. 28.SE - For what amino acids do the following...Ch. 28.SE - Prob. 27APCh. 28.SE - Prob. 28APCh. 28.SE - Draw the complete structure of the ribonucleotide...Ch. 28.SE - Draw the complete structure of the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - Prob. 35APCh. 28.SE - Show the steps involved in a laboratory synthesis...Ch. 28.SE - Draw the structure of cyclic adenosine...Ch. 28.SE - Prob. 38AP
Additional Science Textbook Solutions
Find more solutions based on key concepts
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
The validity of a scientific law.
Physical Universe
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Reagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4- 1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution. Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because they will be used to calibrate the atomic absorption spectrometer. Table 1: Dilutions of Zinc Solutions Solution Zinc Solution Volume Diluted Solution Concentration used volume (ppm Zn) (mL) (mL) concentration (ppm Zn) Solution concentration A 1000 5.00 50.00 1.00×10² (ppm Zn(NO3)2) 2.90×10² Solution concentration (M Zn(NO3)2 1.53×10-3 B Solution A 5.00 100.00 5.00 C Solution B 5.00 50.00 0.50 7.65×10-6 D Solution B 10.00 50.00…arrow_forwardNonearrow_forwardNonearrow_forward
- Nonearrow_forward(b) Provide the number of peaks in each of the indicated signals ('H NMR) for the compound below. CH3 6 1 H&C. C H₂ H2 3 HA 2 2 4 5 5arrow_forward8. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the ground state from higher energy states. Line A has a wavelength of 10.8 nm. BA Increasing wavelength, \ - a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B? b) Identify the one-electron species that exhibits the spectrum.arrow_forward
- Show work with explanation....don't give Ai generated solutionarrow_forwardachieve.macmillanlearning.com Canvas EA eac h Hulu YouTube G 3 methyl cyclobutanol - Google Search Ranking Phenol Acidity Course -236 - Organic Chemistry - Mac... ← Assessment Completed 10 of 22 Questions 1 + Netflix paramount plus chem hw Galdehyde reaction with grignard reagent... b My Questions | bartleby M Inbox - chenteislegit@gmail.com - Gmail Due: Fri, Jan 31 Resources Solution Penalized ? Hint Submit Answer Use retrosynthetic analysis to suggest two paths to synthesize 2-methyl-3-hexanol using the Grignard reaction. (Click and drag the appropriate image to the correct position in the reactions.) Route 1 Aldehyde 1 or +98 Aldehyde 2 Route 2 Q6 +100 Solved in 1 attempt Q7 +95 Solved in 2 attempts Q8 +98 Unlimited attempts possible + + Grignard 1 OH H3O+ Grignard 2 Answer Bank Q9 +90 MgBr Unlimited attempts possible CH3CH2CH2MgBr Q10 Unlimited attempts Q11 ? ? +100 in 1 attempt 2-methyl-3-hexanol CH3CH2MgBr H H о H Attempt 3arrow_forward2) (4 pt) After the reaction was completed, the student collected the following data. Crude product data is the data collected after the reaction is finished, but before the product is purified. "Pure" product data is the data collected after attempted purification using recrystallization. Student B's data: Crude product data "Pure" product data after recrystallization Crude mass: 0.93 g grey solid Crude mp: 96-106 °C Crude % yield: Pure mass: 0.39 g white solid Pure mp: 111-113 °C Pure % yield: a) Calculate the crude and pure percent yields for the student's reaction. b) Summarize what is indicated by the crude and pure melting points.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY