
(a)
Interpretation:
Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with ultraviolet light. Compound C was obtained as a product instead of B.
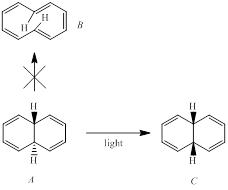
The reason for compound B might be expected to be unstable in spite of its cyclic array of 4n+2 π electrons is to be stated.
Concept introduction:
The stability of
(b)
Interpretation:
The formation of compound B is allowed by the pericyclic selection rules is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The word ‘pericyclic’ means around the cycle. Notably, the pericyclic reactions are classified as electrocyclic, cycloaddition and sigmatropic reactions based on the activation of reaction, the number of electrons involved and the stereochemistry outcome of the reactions. The selection rules of pericyclic reaction deal mostly on the
(c)
Interpretation:
The formation of the observed product C is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The selection rules of pericyclic reaction deal mostly on the rates of reaction and does not tell anything about position of equilibrium and equilibrium constant values.
Trending nowThis is a popular solution!

Chapter 28 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Draw the products formed when the following alkene is treated with 03 followed by Zn, H₂O. Click the "draw structure" button to launch the drawing utility. draw structure ...arrow_forwardCalculate the pH of 0.600 M solution of CH5N (Kb=4.37 x10-4) Hint: use assumption and check it!arrow_forwardDraw all stereoisomers formed when the following alkene is treated with mCPBA. Be sure to answer all parts. Part 1: How many stereoisomers of the product are possible? 1 Part 2 out of 2 Draw the product of the reaction, including stereochemistry. edit structure ...arrow_forward
- Draw the products formed when the following alkene is treated with 03 followed by Zn, H₂O. Be sure to answer all parts. draw structure ... smaller molar mass product draw structure ... larger molar mass productarrow_forwardComplete the two step reaction show the mechanism for all steps.arrow_forwardIdentify whether the reaction would proceed as a E1 or E2 mechanism.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





