
Concept explainers
(a)
Interpretation:
In
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
In
Explanation of Solution
The structure of
This molecule contains seven carbon atoms. In case of the odd number of carbon atoms chain which is in conjugation, the molecular orbitals do not separate out into two equal halves in bonding and antibonding molecular orbitals. Along with that it gives rise to one molecular orbital whose energy is equal to that of
In
(b)
Interpretation:
Each molecular orbital of
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
The molecular orbitals
Explanation of Solution
In molecular orbital theory, the MO is said to be symmetric or anti-symmetric depending on the relative phase of the two terminal carbons. In symmetric MO, the peaks reflect across the reference plane into the peaks and troughs reflect into troughs. On the other hand, in antisymmetric MO, the peaks reflect into troughs and vice versa. According to the general principle, the even number molecular orbitals are antisymmetric and odd number molecular orbitals are symmetric. Therefore, the molecular orbitals
The molecular orbitals
(c)
Interpretation:
The carbon on which the positive charge is delocalized is to be stated. The explanation on the basis of resonance structures and molecular orbital arguments is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. Most of the organic structures cannot be represented using a single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. The delocalization of electrons results in the formation of resonance structure.
Answer to Problem 28.4P
The positive charge is delocalized over
Explanation of Solution
The resonance structure of
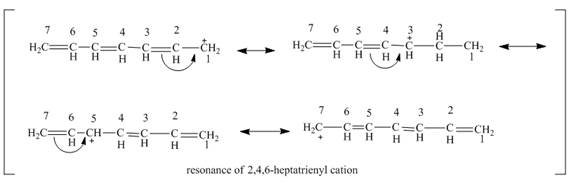
Figure 1
The resonance structures of
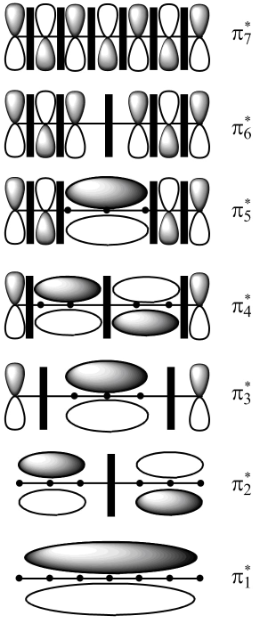
Figure 2
According to the general principle if the node is passing through the carbon then the positive charge is not present on that carbon. In this molecular orbital diagram, the node is passing through the
The positive charge is delocalized over
Want to see more full solutions like this?
Chapter 28 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
