
To calculate: The concentration of each reactant as the function of distance by using the finite difference approach, and apply centred finite-difference approximations with
Answer to Problem 15P
Solution:
The concentration of each reactant as the function of distance is,
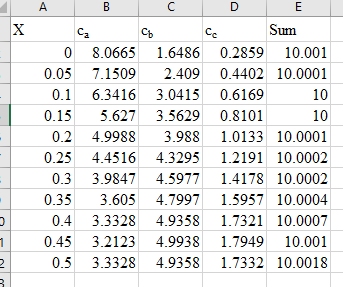
The below plot shows the distance versus reactant.
Explanation of Solution
Given Information:
The series of first order, liquid phase reactions is,
The second order ODEs by using the steady-state mass balance.
Here,
Refer to the Prob 28.14, the Danckwerts boundary conditions is,
Here,
Formula used:
The finite divided difference formula is,
Calculation:
Recall the ordinary differential equations,
Substitute the finite divided difference formula in the above differential equations.
Substitute
Solve further,
Now solve for inlet node
Here use the second order version from the Table 19.3 for the interior nodes,
Can be solved for,
Substitute
Solve for the outer node
The similar equations can be written for the other nodes, because the condition does not include reaction rates Substitute all the parameter gives,
Rearrange the all equations in matrix form for each reactant separately, because the reactions are in series.
Write for the reactant A.
Write the following code in MATLAB.
-
The output is,
Write the all the above equations in matrix form for the reactant B.
Write the following code in MATLAB.
-
The output is,
Write the all the above equations in matrix form for the reactant C.
Write the following code in MATLAB.
-
The output is,
The reaction is in series, thus the system for each reactant is,

The below plot shows the distance versus reactant.
Want to see more full solutions like this?
Chapter 28 Solutions
Numerical Methods for Engineers
- Use the method of undetermined coefficients to solve the given nonhomogeneous system. dx dt = 2x + 3y − 8 dy dt = −x − 2y + 6 X(t) =arrow_forwardAs discussed in Section 8.3, the Markowitz model uses the variance of the portfolio as the measure of risk. However, variance includes deviations both below and above the mean return. Semivariance includes only deviations below the mean and is considered by many to be a better measure of risk. (a) Develop a model that minimizes semivariance for the Hauck Financial data given in the file HauckData with a required return of 10%. Assume that the five planning scenarios in the Hauck Financial Services model are equally likely to occur. Hint: Modify model (8.10)–(8.19). Define a variable ds for each scenario and let ds ≥ R − Rs with ds ≥ 0. Then make the objective function: Min 1 5 5 s = 1 ds2. Let FS = proportion of portfolio invested in the foreign stock mutual fund IB = proportion of portfolio invested in the intermediate-term bond fund LG = proportion of portfolio invested in the large-cap growth fund LV = proportion of portfolio invested in the large-cap value fund…arrow_forwardFor each month of the year, Taylor collected the average high temperatures in Jackson, Mississippi. He used the data to create the histogram shown. Which set of data did he use to create the histogram? A 55, 60, 64, 72, 73, 75, 77, 81, 83, 91, 91, 92\ 55,\ 60,\ 64,\ 72,\ 73,\ 75,\ 77,\ 81,\ 83,\ 91,\ 91,\ 92 55, 60, 64, 72, 73, 75, 77, 81, 83, 91, 91, 92 B 55, 57, 60, 65, 70, 71, 78, 79, 85, 86, 88, 91\ 55,\ 57,\ 60,\ 65,\ 70,\ 71,\ 78,\ 79,\ 85,\ 86,\ 88,\ 91 55, 57, 60, 65, 70, 71, 78, 79, 85, 86, 88, 91 C 55, 60, 63, 64, 65, 71, 83, 87, 88, 88, 89, 93\ 55,\ 60,\ 63,\ 64,\ 65,\ 71,\ 83,\ 87,\ 88,\ 88,\ 89,\ 93 55, 60, 63, 64, 65, 71, 83, 87, 88, 88, 89, 93 D 55, 58, 60, 66, 68, 75, 77, 82, 86, 89, 91, 91\ 55,\ 58,\ 60,\ 66,\ 68,\ 75,\ 77,\ 82,\ 86,\ 89,\ 91,\ 91 55, 58, 60, 66, 68, 75, 77, 82, 86, 89, 91, 91arrow_forward
- 3. Consider the polynomial equation 6-iz+7z2-iz³ +z = 0 for which the roots are 3i, -2i, -i, and i. (a) Verify the relations between this roots and the coefficients of the polynomial. (b) Find the annulus region in which the roots lie.arrow_forwardc) Using only Laplace transforms solve the following Samuelson model given below i.e., the second order difference equation (where yt is national income): - Yt+2 6yt+1+5y₁ = 0, if y₁ = 0 for t < 0, and y₁ = 0, y₁ = 1 1-e-s You may use without proof that L-1[s(1-re-s)] = f(t) = r² for n ≤tarrow_forwardScoring: MATH 15 FILING /10 COMPARISON /10 RULER I 13 Express EMPLOYMENT PROFESSIONALS NAME: SKILLS EVALUATION TEST- Light Industrial MATH-Solve the following problems. (Feel free to use a calculator.) DATE: 1. If you were asked to load 225 boxes onto a truck, and the boxes are crated, with each crate containing nine boxes, how many crates would you need to load? 2. Imagine you live only one mile from work and you decide to walk. If you walk four miles per hour, how long will it take you to walk one mile? 3. Add 3 feet 6 inches + 8 feet 2 inches + 4 inches + 2 feet 5 inches. 4. In a grocery store, steak costs $3.85 per pound. If you buy a three-pound steak and pay for it with a $20 bill, how much change will you get? 5. Add 8 minutes 32 seconds + 37 minutes 18 seconds + 15 seconds. FILING - In the space provided, write the number of the file cabinet where the company should be filed. Example: File Cabinet #4 Elson Co. File Cabinets: 1. Aa-Bb 3. Cg-Dz 5. Ga-Hz 7. La-Md 9. Na-Oz 2. Bc-Cf…arrow_forwardIf you were asked to load 225 boxes onto a truck, and the boxes are crated, with each crate containing nine boxes, how many crates would you need to load?arrow_forwardHabitat for Humanity International is a nonprofit organization dedicated to eliminating poverty housing worldwide. Suppose the following table contains estimates of activity times (in days) involved in the construction of a house that Habitat for Humanity is building. Activity Optimistic Most Probable Pessimistic A 6 7.0 8 B 7 8.0 9 C 7 7.5 11.5 D 7 9.0 10 E 6 7.0 9 F 3 4.0 5 (a) Compute the expected activity completion times and the variance for each activity. (Round your answers to two decimal places.) Activity Expected Times A B C D E F Variance (b) An analyst determined that the critical path consists of activities B-D-F. Compute the expected project completion time and the variance of this path. (Round your answers to two decimal places.) expected project completion time variance of projection completion timearrow_forwardTo manage the production of an animated movie, Pixar Animation Studios has listed the major activities involved, the predecessor relationships, and activity times (in months). The project is completed when activities F and G are both complete. Activity Immediate Predecessor G A B CD E A A C, B C, B D, E Time 4 6 2 6 3 3 5 (a) Find the critical path. (Enter your answers as a comma-separated list.) (b) The project must be completed in 1.5 years. Do you anticipate difficulty in meeting the deadline? Explain. The critical path activities require months to complete. Thus the project ---Select--- be completed in 1.5 years.arrow_forwardTo help with preparations, a couple has devised a project network to describe the activities that must be completed by their wedding date. In addition, they have estimated the time of each activity (in weeks). Start D F B E G Activity A B C DEFGH Time 5 3 6 6 6 3 11 10 (a) Identify the critical path. (Enter your answers as a comma-separated list.) H Finish (b) How much time (in weeks) will be needed to complete this project? week(s) (c) Can activity D be delayed without delaying the entire project? If so, by how many weeks? (If the activity can not be delayed, enter 0.) week(s) (d) Can activity C be delayed without delaying the entire project? If so, by how many weeks? (If the activity can not be delayed, enter 0.) week(s) (e) What is the schedule for activity E (in weeks)? Earliest Start Latest Start Earliest Finish Latest Finish week(s) week(s) week(s) week(s)arrow_forward30.6. Classify the zeros and singularities of the functions tanz (a). f(z)=sin(1-2-1), (b). f(2) = (c). f(z)= tanh .arrow_forward1. Locate the singularities of three of the following functions, and determine their type. (a) f(z)=2(z-sinz). (b) f(z) = (-) (c) f(z) = (z+2-22²)-1 (d) f(z) = sinzarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated
Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education
Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY
Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,
Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,






