
Concept explainers
(a)
Interpretation:
The principal product formed when
Concept introduction:
The general mechanism of electrophilic addition is as follows:
Step1:
Step2: Then the nucleophilic part of reagent adds to the carbocation to give the addition product.
These steps can be illustrated as follows:
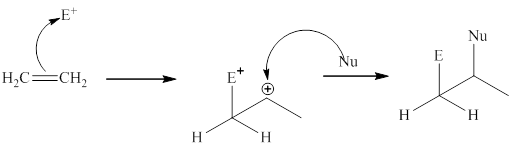
(b)
Interpretation:
The principal product formed in the monochlorination of propane should be predicted and written.
Concept introduction:
The reaction between chlorine and propane under ultraviolet light is the monohalogenation reaction. This is essentially a substitution reaction. Every possible monochlorinated products are formed. The major one is the one where chlorine is attached at the terminal carbon.
The principal product in the halogenation of
(c)
Interpretation:
The principal product when isopropyl alcohol is heated with benzoic acid should be predicted and written.
Concept introduction:
Heating alcohol and acid involve esterification reaction and an ester is formed along with the release of a water molecule.
The reaction can be illustrated as:

Where
The general mechanistic pathway for esterification as proposed by Fischer is as follows:
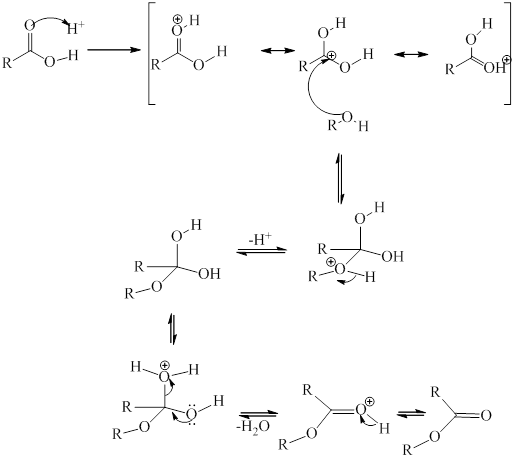
(d)
Interpretation:
The principal product when
Concept introduction:
Oxidation of alcohols can be done with an oxidizing reagent.
Tertiary alcohols cannot be oxidized as they do not have hydrogen; the secondary alcohols are oxidized to
While the primary alcohols oxidized to
The general formula of a tertiary, secondary and primary alcohol is represented as follows:
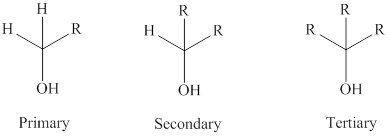
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
General Chemistry: Principles And Modern Applications Plus Mastering Chemistry With Pearson Etext -- Access Card Package (11th Edition)
- [In this question, there are multiple answers to type in a "fill-in-the-blank" fashion - in each case, type in a whole number.] Consider using Slater's Rules to calculate the shielding factor (S) for the last electron in silicon (Si). There will be electrons with a 0.35 S-multiplier, electrons with a 0.85 S-multiplier, and electrons with a 1.00 S-multiplier.arrow_forwardProvide the unknown for the given data.arrow_forwardDraw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forward
- Steps and explanation please.arrow_forwardHow could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forward
- Provide the unknown for the given dataarrow_forwardProvide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





