
Concept explainers
Describe what is meant by each of the following reaction types, and illustrate with an example:
(a) nucleophilic substitution reaction: (b) electrophilic substitution reaction; (c) addition reaction;
(d) elimination reaction, (e) rearrangement reaction.
(a)
Interpretation:
The nucleophilic substitution reaction should be defined with example.
Concept introduction:
Nucleophilic substitution reaction describes the attack of the electron-rich group that is nucleophile on electron deficient groups that is electrophile.
Answer to Problem 1E
Nucleophilic substitution reaction is the type of reaction in which the nucleophile (electron rich species) attacks the electron-deficient carbon atom which is electrophilic.
Explanation of Solution
Nucleophilic substitution reaction is defined as an organic reaction which includes the attack of a nucleophile on electrophilic center along with the removal of the leaving group.
The example of the nucleophilic substitution reaction is,
In this reaction, chlorine of chloroethane is replaced by a hydroxyl group
(b)
Interpretation:
The electrophilic substitution reaction should be defined with example.
Concept introduction:
The electrophilic substitution reaction describes the displacement of functional group or hydrogen atom by an electron deficient group or electrophile.
Answer to Problem 1E
Electrophilic substitution reaction is defined as the organic reaction in which the electrophile replaces a functional group of a compound or hydrogen atom.
Explanation of Solution
Electrophilic substitution reaction is defined as the organic reaction which includes the replacement of functional group or
Example of electrophilic substitution reaction is,
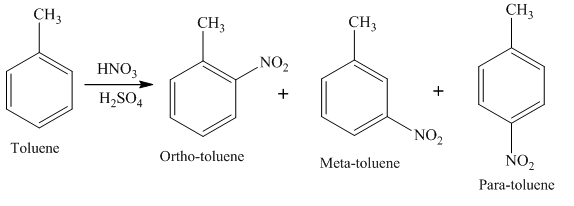
In this reaction, Toluene undergoes electrophilic substitution to form para nitrotoluene, meta nitrotoluene, and ortho nitrotoluene.
(c)
Interpretation:
The addition reaction should be defined with example.
Concept introduction:
The addition reaction describes the combination of two or more smaller molecules to form a larger molecule.
Answer to Problem 1E
Addition reaction is defined as the reaction in which two or more molecules combine to form a single and large molecule.
Explanation of Solution
The reaction of the addition of the two or more reactants that is A and B to produce a single product C is termed as addition reaction.
The example of addition reaction is,
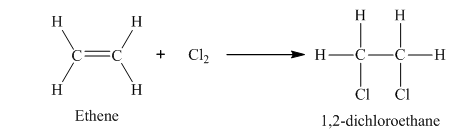
In this reaction, chlorine molecule combines with ethene to form 1, 2-dichloroethane.
(d)
Interpretation:
The elimination reaction should be defined with example.
Concept introduction:
The elimination reaction describes the removal of two substituents from the reactant molecule to form the product.
Answer to Problem 1E
Elimination reaction is the reaction by which the reactant molecule or compound breaks into two or more products.
Explanation of Solution
Elimination reaction is the type of reaction in which two substituents are removed from the reactant molecule to form the product. Generally, unsaturated compounds are formed in an elimination reaction.
The example of the elimination reaction is,
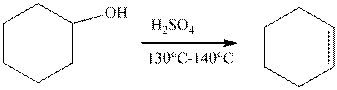
The reaction of cyclohexanol in the presence of
(e)
Interpretation:
The rearrangement reaction should be defined with example.
Concept introduction:
The rearrangement reaction describes the rearrangement of bonds in a molecule to form the product.
Answer to Problem 1E
It is the process of movement of bonds within a molecule to give rise to structural isomers.
Explanation of Solution
It is defined as a reaction in which an atom or a bond migrates from one atom in reactant molecule to adjacent atom to give rise to the product.
Example of rearrangement reaction is,
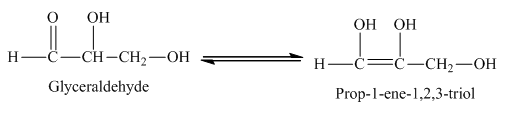
The glyceraldehyde undergoes rearrangement to form enediol.
Want to see more full solutions like this?
Chapter 27 Solutions
General Chemistry: Principles And Modern Applications Plus Mastering Chemistry With Pearson Etext -- Access Card Package (11th Edition)
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Organic Chemistry
Physical Universe
Campbell Biology: Concepts & Connections (9th Edition)
General, Organic, and Biological Chemistry - 4th edition
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
- Part VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward
- 13C NMR is good for: a) determining the molecular weight of the compound b) identifying certain functional groups. c) determining the carbon skeleton, for example methyl vs ethyl vs propyl groups d) determining how many different kinds of carbon are in the moleculearrow_forward6 D 2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel. Could you have performed this experiment using hexane instead of water? Explain. 3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained from the steam distillation of orange peel.arrow_forwardPart III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





