
(a)
Interpretation:
The main product of the mononitration of benzoic acid should be predicted.
Concept introduction:
The electrophilic
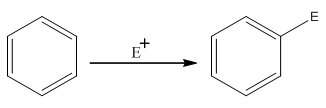
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The nitration reaction takes place in the presence of nitric acid and sulphuric acid. In this reaction, the protonation of nitric acid occurs in order to produce the nitronium ion. The nitronium ion will attach on the benzene ring to form nitrobenzene. The general reaction is as follows:
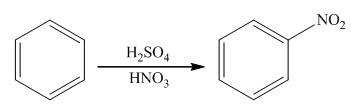
(b)
Interpretation:
The main product of the monosulphonation of phenol should be predicted.
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
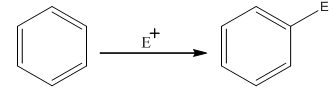
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The sulfonation takes place in the presence of sulphuric acid. In this reaction, sulfur trioxide is formed that acts as an electrophile. Sulfur trioxide will attach on the benzene ring to form the final product. The general reaction is as follows:
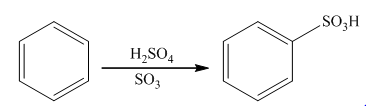
(c)
Interpretation:
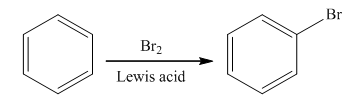
The main product of the monobromination of
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
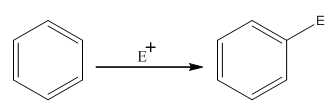
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
Halogenation is the
The bromination takes place in the presence of Lewis acid and bromine molecule. In this reaction, the bromonium ion is produced that acts as an electrophile. Brominium will attach on the benzene ring to form the final product. The general reaction is as follows:
Trending nowThis is a popular solution!

Chapter 27 Solutions
EP GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
- Part C IN H N. Br₂ (2 equiv.) AlBr3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and + e (×) H± 12D T EXP. L CONT. דarrow_forward9. OA. Rank the expected boiling points of the compounds shown below from highest to lowest. Place your answer appropriately in the box. Only the answer in the box will be graded. (3) points) OH OH بر بد بدید 2 3arrow_forwardThere is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS). Ca, ppm V, ppm SCa, arb. units SV, arb. units 20.0 10.0 14375.11 14261.02 40.0 10.0 36182.15 17997.10 60.0 10.0 39275.74 12988.01 80.0 10.0 57530.75 14268.54 100.0…arrow_forward
- A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C. H₂O(g) + C₁₂O(g) = 2 HOCl(g) K = 0.0900 at 25°C с Calculate the equilibrium concentrations of each gas at 25 °C. [H₂O]= [C₁₂O]= [HOCI]= M Σ Marrow_forwardWhat units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forward
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

