
EP GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
11th Edition
ISBN: 9780133897340
Author: Petrucci
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 16E
Substitution and Elimination Reactions
Molecule (a) below reacts faster in the SN1 reaction than does molecule (b). Explain this observation [Hint: Draw chair structures for the two molecules.]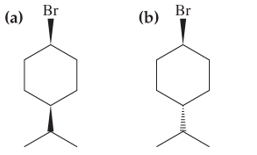
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution,
respectively.
F CI
Br |
Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to
have a reasonable yield of product.
NH2
Br
Br
Br
OH
Br
Q7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability.
a) H₂O, OH, CH3COOT
b) NH3, H₂O, H₂S
Q8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution
reaction with CN as the nucleophile.
Br
A
B
NH2
LL
F
C
D
OH
CI
LLI
E
Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants,
and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for
reactions a) to d).
a)
H
"Cl
D
+
-OCH 3
Page 3 of 5
Chapter 27 Solutions
EP GENERAL CHEMISTRY-MOD.MASTERINGCHEM.
Ch. 27 - Describe what is meant by each of the following...Ch. 27 - Prob. 2ECh. 27 - Prob. 3ECh. 27 - Prob. 4ECh. 27 - Prob. 5ECh. 27 - Prob. 6ECh. 27 - Prob. 7ECh. 27 - Prob. 8ECh. 27 - Answer the following questions for this SN2...Ch. 27 - Answer the following questions for this SN1...
Ch. 27 - Substitution and Elimination Reactions Answer the...Ch. 27 - Prob. 12ECh. 27 - Prob. 13ECh. 27 - Prob. 14ECh. 27 - Prob. 15ECh. 27 - Substitution and Elimination Reactions Molecule...Ch. 27 - Prob. 17ECh. 27 - Prob. 18ECh. 27 - Prob. 19ECh. 27 - Prob. 20ECh. 27 - Prob. 21ECh. 27 - Prob. 22ECh. 27 - Prob. 23ECh. 27 - Substitution and Elimination Reactions (R) — 2 —...Ch. 27 - Prob. 25ECh. 27 - Prob. 26ECh. 27 - Prob. 27ECh. 27 - Prob. 28ECh. 27 - Prob. 29ECh. 27 - Alcohols and Alkenes Predict the product(s) of the...Ch. 27 - Prob. 31ECh. 27 - Alcohols and Alkenes Give the structure of the...Ch. 27 - Alcohols and Alkenes Give the major product that...Ch. 27 - Prob. 34ECh. 27 - Prob. 35ECh. 27 - Prob. 36ECh. 27 - Prob. 37ECh. 27 - Prob. 38ECh. 27 - Prob. 39ECh. 27 - Prob. 40ECh. 27 - Prob. 41ECh. 27 - Prob. 42ECh. 27 - Reactions of Alkanes (a) Write the initiation,...Ch. 27 - Reactions of Alkanes Write the initiation,...Ch. 27 - Prob. 45ECh. 27 - Polymerization Reactions Explain why Dacron is...Ch. 27 - Prob. 47ECh. 27 - Prob. 48ECh. 27 - Prob. 49ECh. 27 - Prob. 50ECh. 27 - Prob. 51ECh. 27 - Prob. 52ECh. 27 - Prob. 53ECh. 27 - Prob. 54ECh. 27 - Prob. 55IAECh. 27 - Prob. 56IAECh. 27 - Prob. 57IAECh. 27 - Prob. 58IAECh. 27 - Prob. 59IAECh. 27 - Prob. 60IAECh. 27 - Prob. 61IAECh. 27 - Prob. 62IAECh. 27 - Prob. 63IAECh. 27 - Prob. 64IAECh. 27 - Prob. 65IAECh. 27 - Prob. 66IAECh. 27 - Prob. 67IAECh. 27 - Prob. 68IAECh. 27 - Prob. 69IAECh. 27 - Prob. 70IAECh. 27 - Prob. 71IAECh. 27 - Prob. 72IAECh. 27 - Prob. 73IAECh. 27 - Prob. 74IAECh. 27 - Prob. 75IAECh. 27 - Prob. 76IAECh. 27 - Prob. 77IAECh. 27 - The reduction of aldehydes and ketones with a...Ch. 27 - Explain the important distinctions between each...Ch. 27 - Prob. 80SAECh. 27 - Prob. 81SAECh. 27 - Prob. 82SAECh. 27 - Prob. 83SAECh. 27 - Prob. 84SAECh. 27 - What is the major organic product obtained in the...Ch. 27 - Prob. 86SAE
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
The validity of a scientific law.
Physical Universe
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
- Explain why only the lone pairs on the central atom are taken into consideration when predicting molecular shapearrow_forward(ME EX1) Prblm #9/10 Can you explain in detail (step by step) I'm so confused with these problems. For turmber 13 can u turn them into lewis dot structures so I can better understand because, and then as well explain the resonance structure part. Thanks for the help.arrow_forwardProblems 19 and 20: (ME EX1) Can you please explain the following in detail? I'm having trouble understanding them. Both problems are difficult for me to explain in detail, so please include the drawings and answers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License