
Concept explainers
Interpretation: To identify the correct pairing of standard amino acid carbon skeleton degradation product and amino acid genicity.
Concept introduction: Genicity of an amino acid is defined as whether the carbon skeleton degradation product of an amino acid can produce glucose or
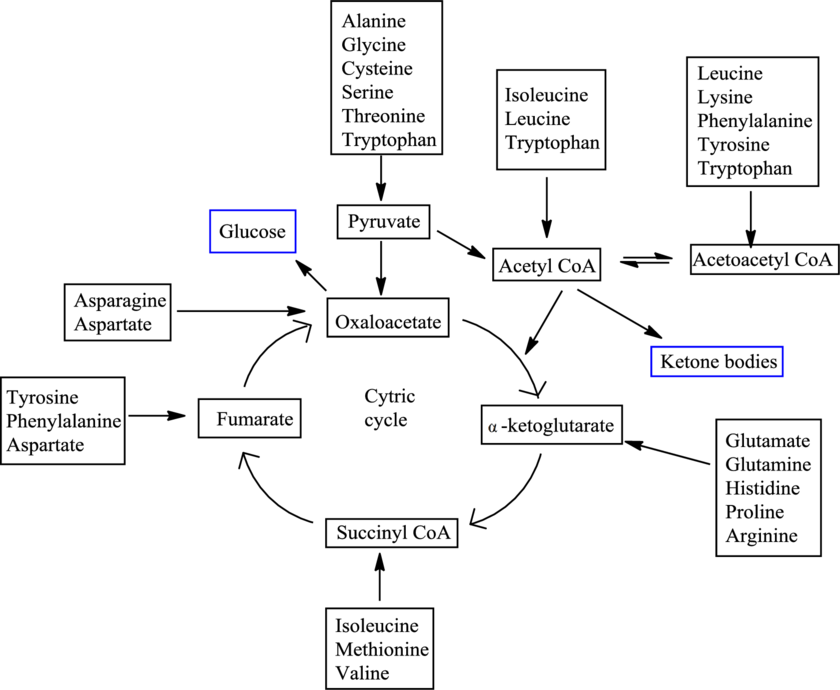
There are 20 standard amino acids and each has a different carbon skeleton. An amino acid is known as a glucogenic amino acid if its carbon-containing degradation product can be used to produce glucose. An amino acid is known as a ketogenic amino acid if its carbon-containing degradation product can be used to produce ketone bodies.
The degradation pathways for different amino acids merge in between and result in the formation of only 7 products. The 7 products are
Fates of carbon skeletons of different amino acids are as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



