
(a)
Interpretation:
The synthesis of the given product from pyridine is to be predicted.
Concept introduction:
The replacement or substitution of one
Answer to Problem 26.39AP
The synthesis for the given product from pyridine is shown below.
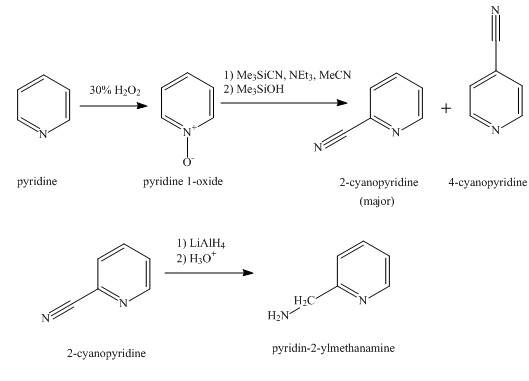
Explanation of Solution
In the first step, pyridine undergoes oxidation on nitrogen atom to form pyridine-N-oxide on reaction with 30 % H2O2 solution.
In the second step, the treatment of pyridine-N-oxide with Me3SiCN, Et3N, MeCN followed by Me3SiOH occurs and it undergoes a nucleophilic
In the final step, the reduction of 2-cyanopyridine with the help of lithium aluminum hydride takes place to form the given product. The steps of conversion are shown below.

Figure 1
The synthesis for the given product from pyridine is shown in Figure 1.
(b)
Interpretation:
The synthesis of the given product from pyridine is to be predicted.
Concept introduction:
The formation of diazonium salt from aromatic
The addition of the nitro group is known as nitration.
Answer to Problem 26.39AP
The synthesis for the given product from pyridine is shown below.
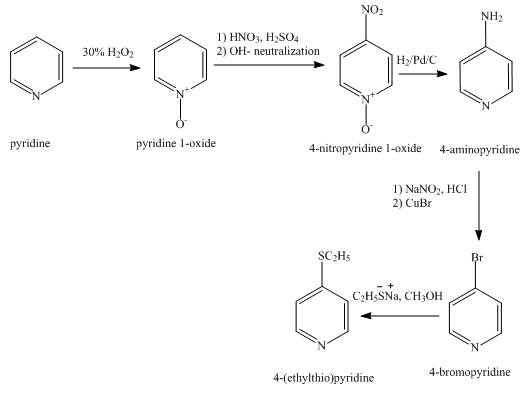
Explanation of Solution
In the first step, pyridine undergoes oxidation on nitrogen atom to form pyridine-N-oxide on reaction with 30 % H2O2 solution. In the second step, pyridine-N-oxide with the nitric acid undergoes nitration reaction at the fourth position to form 4-nitropyridine-N-oxide.
In the third step, the reduction of 4-nitropyridine-N-oxide takes place with the help of H2, Pd/C in which nitro group and N-oxide group gets reduced.
In the fourth step, 4-aminopyridine reacts with NaNO2, HCl followed by reaction with CuBr and gives 4-bromopyridine.
In the fifth step, 4-bromopyridine with sodium ethylthiolate in presence of methanol undergoes nucleophilic substitution to form the desired product.
The overall reaction is shown below.

Figure 2
The synthesis for the given product from pyridine is shown in Figure 2.
(c)
Interpretation:
The synthesis of the given product from furfural is to be predicted.
Concept introduction:
Grignard reagents are
Answer to Problem 26.39AP
The synthesis of the given product from furfural is shown below.
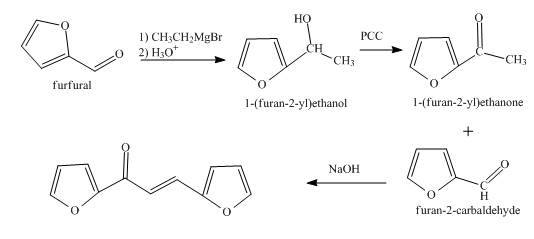
Explanation of Solution
In the first step, the treatment of fufural with CH3MgBr followed by acid hydrolysis takes place.
In the second step, the product obtained with the help of pyridinium chlorochromate undergoes oxidation to form the corresponding
In the final step, methyl ketone reacts with the furfural in the presence of NaOH solution to form α,β-unsaturated carbonyl compound. This reaction is known as aldol condensation.
The overall reaction is shown below.

Figure 3
The synthesis of the given product from furfural is shown in Figure 3.
(d)
Interpretation:
The synthesis of the given product from furfural is to be predicted.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from furfural is shown below.
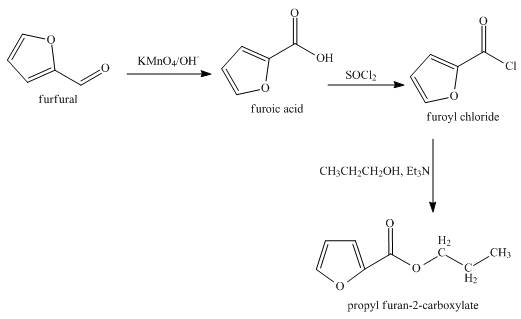
Explanation of Solution
In the first step, the reaction of furfural with KMnO4 solution undergoes oxidation reaction to form furoic acid.
In the second step, furoic reacts acid with thionyl chloride to form furoyl chloride. The type of reaction is nucleophilic acyl substitution reaction.
In the third step, furoyl chloride with 1-propanol in the presence of trimethylamine undergoes esterification to form the desired product.
The overall reaction is shown below.

Figure 4
The synthesis of the given product from furfural is shown in Figure 4.
(e)
Interpretation:
The synthesis of the given product from 3-methylpyridine is to be predicted
Concept introduction:
Oxidation is the process of addition of oxygen. Oxidation is also defined as loss of electrons. In the oxidation process, there is increasing in the oxidation state. The acid is added in the reaction for protonation.
The oxidizing agent is defined as the species which oxidizes others and itself gets reduced.
Answer to Problem 26.39AP
The synthesis of the given product from 3-methylpyridine is shown below.
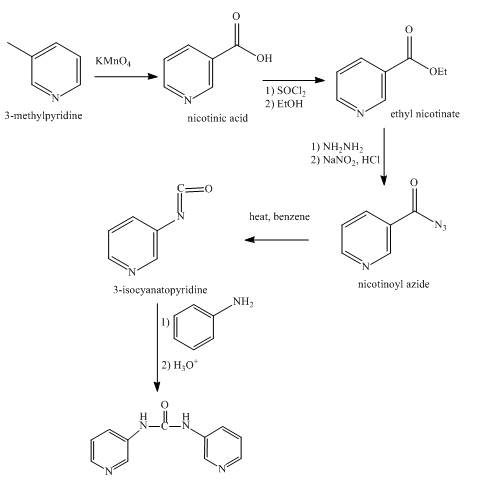
Explanation of Solution
In the first step, 3-methylpyridine with KMnO4 forms nicotine as product.
In the second step, the reaction between nicotinic acid and thionyl chloride takes place in the presence of ethanol and gives the corresponding methyl ester as product.
In the third step, the reaction between methyl ester with hydrazine in the presence of NaNO2, HCl takes place and forms the corresponding acyl azide.
In the fourth step, the acyl azide on heating with benzene gives isocyanate as the product.
In the fifth step, the isocyanate is treated with pyridin-3-amine to give the desired product.

Figure 5
The synthesis of the given product from 3-methylpyridine is shown in Figure 5.
(f)
Interpretation:
The synthesis of the given product from 4-ethylpyridine is to be predicted.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from 4-ethylpyridine is shown below.
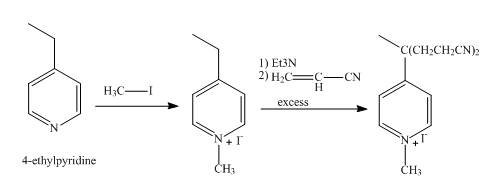
Explanation of Solution
In the first step, the reaction of 4-ethylpyridine with methyl iodide undergoes methylation at nitrogen atom to form N-methyl-4-ethyl pyridinium iodide as product.
In the second step, N-methyl-4-ethyl pyridinium iodide with triethylamine undergoes abstraction of a proton from ethyl carbon of pyridine. The resulting anion is stabilized by resonance with the help of electronegative nitrogen atom, followed by the nucleophilic substitution reaction with the vinyl nitrile to form the desired product.
The overall reaction is shown below.

Figure 6
The synthesis of the given product from 4-ethylpyridine is shown in Figure 6.
(g)
Interpretation:
The synthesis of the given product from 2-methylpyridine is to be predicted.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from 2-methylpyridine is shown below.
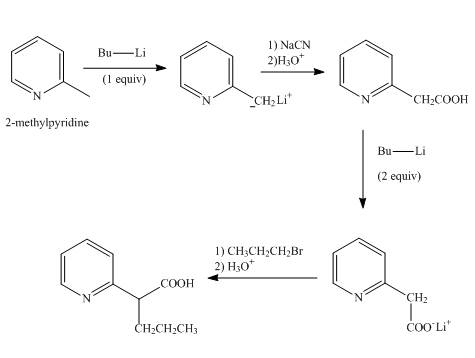
Explanation of Solution
In the first step, 2-methylpyridine with 1 equivalent of butyl lithium undergoes abstraction of proton from methyl group to form resonanace stabilized anion.
In the second step, the treatment of resonance stabilized anion with sodium cyanide followed by hydrolysis gives a
In the third step, the treatment of 2-(pyridin-2-yl)acetic acid with 2 equivalents of butyl lithium undergoes proton abstraction at the methylene carbon to form resonance stabilized anion.
In the final step, the resonance stabilized anion undergoes a nucleophilic substitution reaction with propyl bromide to form the desired product.
The overall reaction is shown below.

Figure 7
The synthesis of the given product from 2-methylpyridine is shown in Figure 7.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





