
Concept explainers
(a)
Interpretation: To determine whether oxaloacetate and
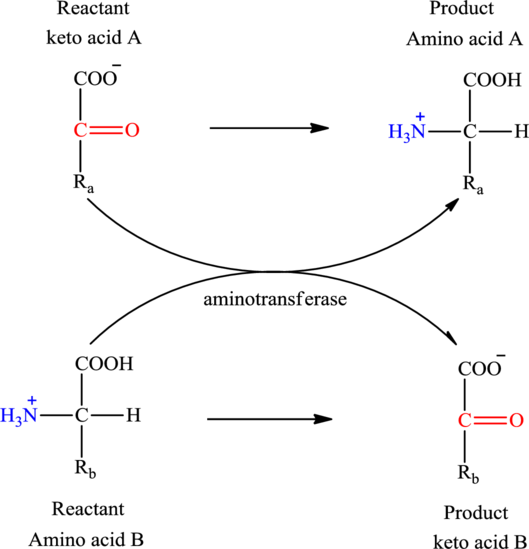
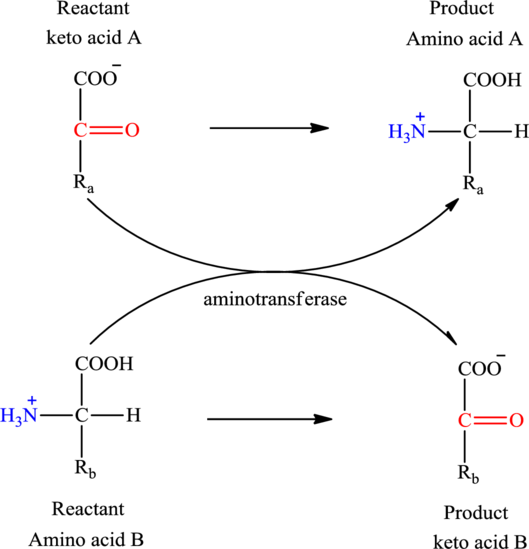
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
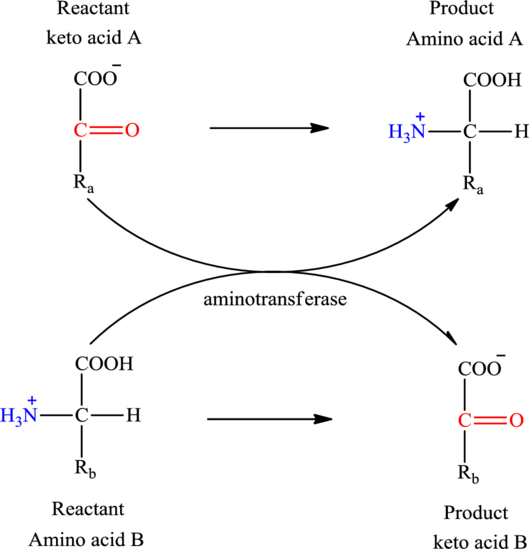
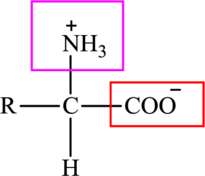
The general structure of an amino acid is:
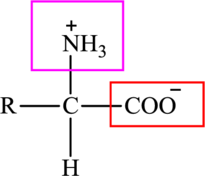
Here,
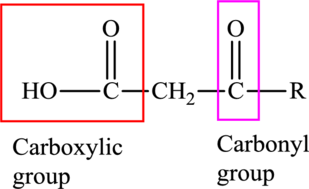
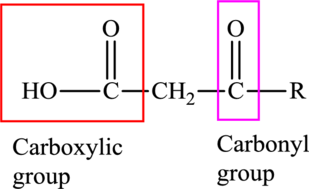
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:
(a)
Answer to Problem 26.33EP
No, oxaloacetate and
Explanation of Solution
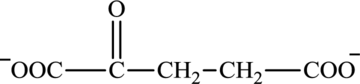
Oxaloacetate is a keto acid and its structure is:
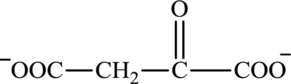
The two reactants in transamination reaction are a keto acid and an amino acid. Both oxaloacetate and
(b)
Interpretation: To determine whether glutamate and oxaloacetate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
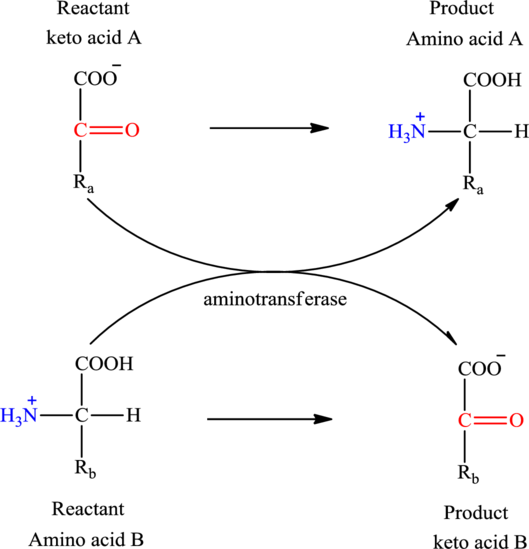
The general structure of an amino acid is:
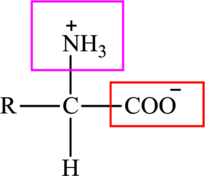
Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:
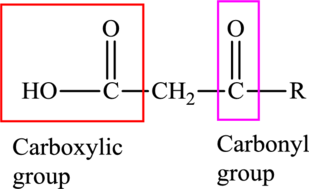
(b)
Answer to Problem 26.33EP
Yes, glutamate and oxaloacetate can function as the reactants in a transamination reaction.
Explanation of Solution
Glutamate is an amino acid and its structure is:
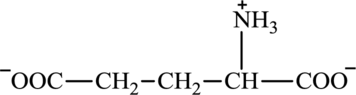
Oxaloacetate is a keto acid and its structure is:
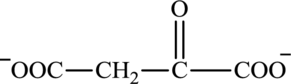
Transamination reaction involves the exchange of an amino group from an
(c)
Interpretation: To determine whether glutarate and glutamate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
The general structure of an amino acid is:
Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:
(c)
Answer to Problem 26.33EP
No, glutarate and glutamate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Glutarate is a diacid and its structure is:
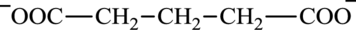
Glutamate is an amino acid and its structure is:
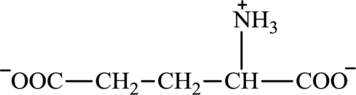
The two reactants in transamination reaction are a keto acid and an amino acid. Glutamate is an amino acid but glutarate is not a keto acid. For a transamination reaction to take place there must be one keto acid present along with an amino acid. Thus, glutarate and glutamate cannot function as the reactants in a transamination reaction.
(d)
Interpretation: To determine whether oxaloacetate and succinate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
The general reaction to illustrate transamination is as follows:
The general structure of an amino acid is:
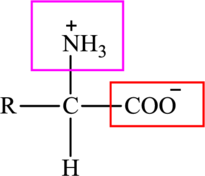
Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:
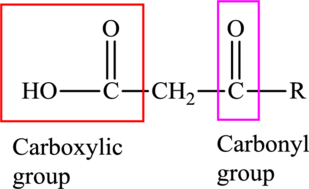
(d)
Answer to Problem 26.33EP
No, oxaloacetate and succinate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Oxaloacetate is a keto acid and its structure is:
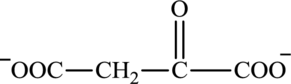
Succinate is a diacid acid and its structure is:
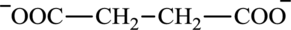
The two reactants in transamination reaction are a keto acid and an amino acid. Oxaloacetate is keto acid but succinate is not an amino acid. For a transamination reaction to take place there must be one amino acid present along with a keto acid. Thus, oxaloacetate and succinate cannot function as the reactants in a transamination reaction.
Want to see more full solutions like this?
Chapter 26 Solutions
General, Organic, and Biological Chemistry Seventh Edition
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



