
(a)
Interpretation:
The structure of quinine in
Concept introduction:
Aliphatic
Answer to Problem 26.33AP
The structure of quinine in
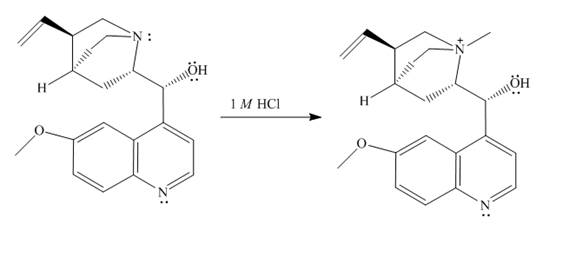
Explanation of Solution
When quinine reacts with
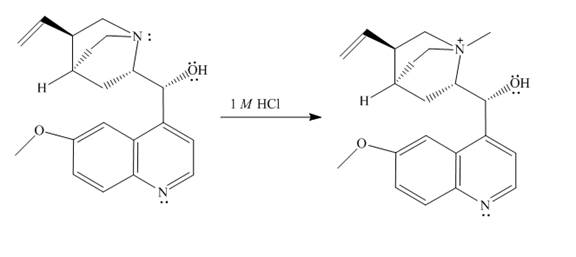
Figure 1
The structure of quinine in
(b)
Interpretation:
The structure of nicotine in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of nicotine in
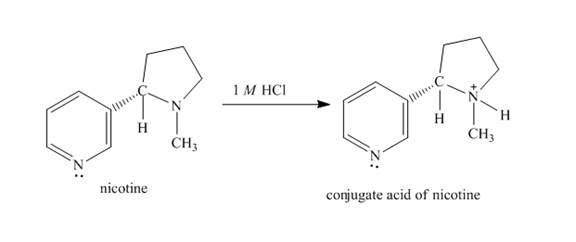
Explanation of Solution
When nicotine reacts with
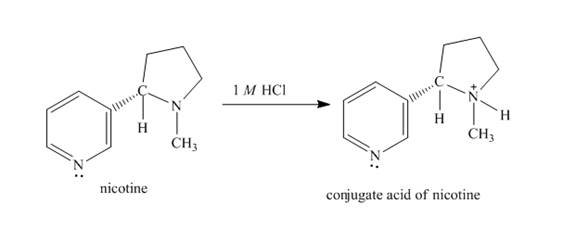
Figure 2
The structure of nicotine in
(c)
Interpretation:
The structure of tryptamine in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of tryptamine in
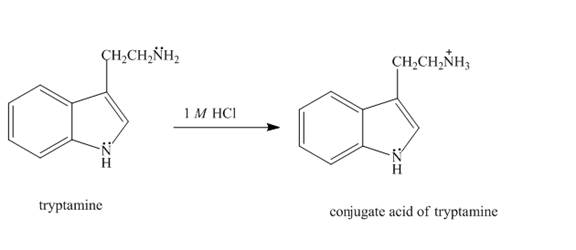
Explanation of Solution
The aliphatic amine present on the side chain of tryptamine gets protonated in

Figure 3
The structure of tryptamine in
(d)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
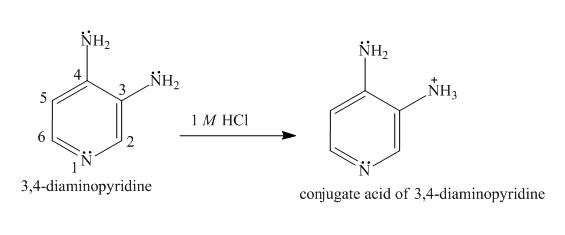
Explanation of Solution
In the structure of

Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
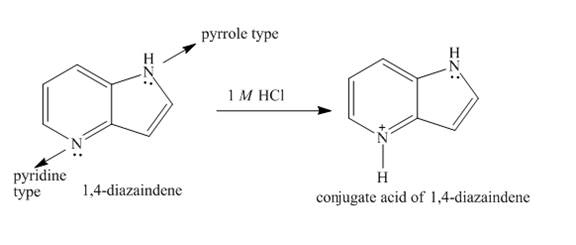
Explanation of Solution
In

Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of
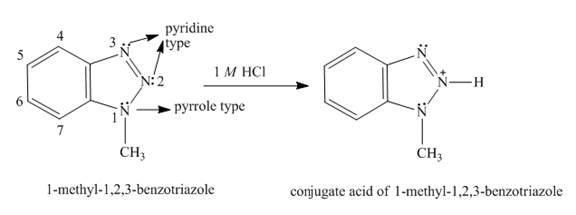
Explanation of Solution
In
The formation of conjugated acid of

Figure 6
The structure of
(g)
Interpretation:
The structure of imitanib in
Concept introduction:
Aliphatic amines are more basic than aromatic amines. In aliphatic amines, the nitrogen atom is attached to a methyl group which is electron donating group; therefore, it increases the electron density on nitrogen by
Answer to Problem 26.33AP
The structure of imitanib in
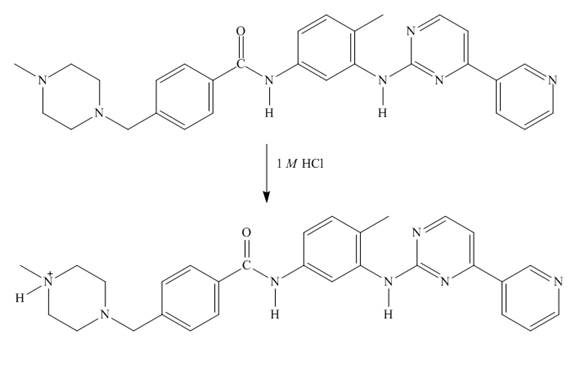
Explanation of Solution
In imitanib, the aliphatic amine will be protonated than aromatic amine since the basicity of aliphatic amines is more. The aliphatic amine present adjacent to the methyl group will be protonated as it is electron rich due to the
Figure 7
The structure of imitanib in
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





