
Concept explainers
(a)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the
Answer to Problem 26.27AP
The product formed when
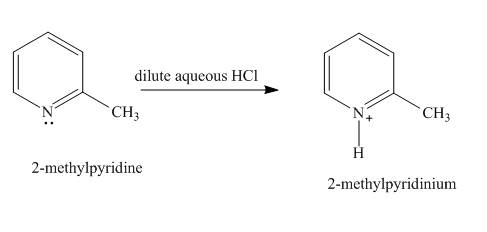
Explanation of Solution
In the given compound, the lone pair of the pyridine nitrogen is not involved in the resonance with the ring. Pyridine acts as base in the presence of acid. The nitrogen abstracts the proton from acid. The product formed is

Figure 1
The product formed in the given reaction is
(b)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. No delocation of electron takes place, hence pyridine acts as a base. The hydrogen atoms of pyridine ring are not highly acidic, so mild base can’t abstract proton from pyridine. It requires a strong base for abstraction of proton.
Answer to Problem 26.27AP
No reaction takes place when
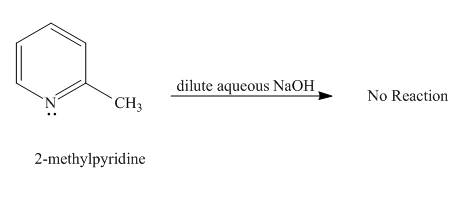
Explanation of Solution
Protons of pyridine are not highly acidic in nature. Sodium hydroxide cannot abstract proton from pyridine. It requires a very strong base for the removal of proton from pyridine to takes place. Hence, no reaction takes place between

Figure 2
No product is formed in the given reaction.
(c)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. No delocation of electron takes place, hence pyridine acts as a base. The hydrogen atoms of pyridine ring are not highly acidic, so mild base can’t abstract proton from pyridine. It requires a strong base for abstraction of proton.
Answer to Problem 26.27AP
The product formed when
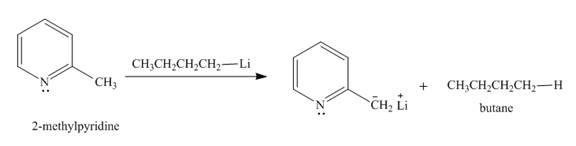
Explanation of Solution
The butyl-lithium compound is a very strong base. It will abstract proton from the methyl group of
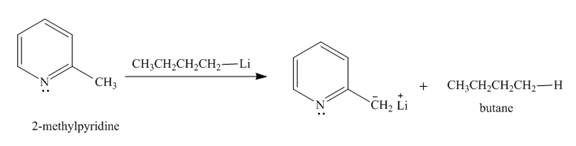
Figure 3
The product formed in the given reaction is
(d)
Interpretation:
The product formed when
Concept Introduction:
Nitration reaction is aromatic electrophilic substitution reaction. The nitrating mixture contains nitric acid and sulfuric acid. The nitrosonium ion is formed as electrophile. The methyl group is an activating group, so it promotes electrophilic substitution reaction at ortho-para position.
Answer to Problem 26.27AP
The product formed when
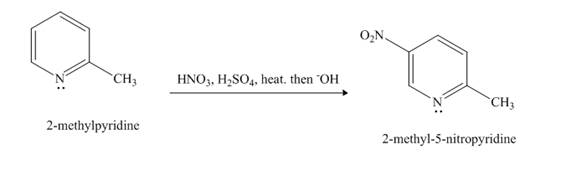
Explanation of Solution
The compound

Figure 4
The product formed in the given reaction is
(e)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. Hydrogen peroxide is a strong oxidizing agent. It will oxidize the nitrogen atom of pyridine to form
Answer to Problem 26.27AP
The product formed when
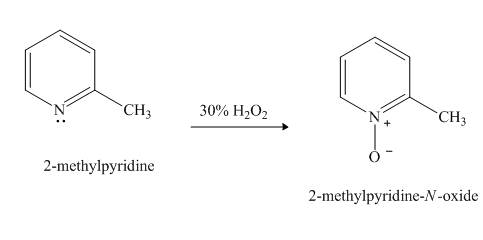
Explanation of Solution
Hydrogen peroxide is an oxidizing agent. It will oxidize the given compound. The oxidation reaction takes place at the nitrogen atom.

Figure 5
The product formed in the given reaction of
(f)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. No delocation of electron takes place, hence pyridine acts as a base. The nitrogen atom lone pair attacks the methyl group of methyl iodide.
Answer to Problem 26.27AP
The product formed when
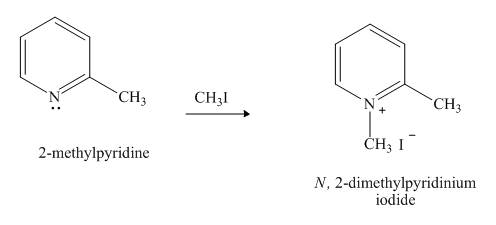
Explanation of Solution
The lone pair of nitrogen atom of pyridine is not involved in the resonance with the aromatic ring. The lone pair makes the pyridine compound basic in nature. When compound

Figure 6
The product formed in the given reaction of
(g)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. No delocation of electron takes place, hence pyridine acts as a base. Lithiated pyridine compound reacts with
Answer to Problem 26.27AP
The product formed when
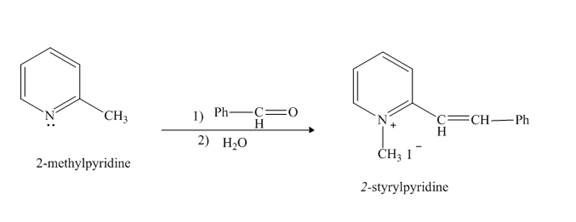
Explanation of Solution
The lithiation product formed reacts with benzaldehyde, nucleophilic addition reaction takes place to form

Figure 7
The product formed when
(h)
Interpretation:
The product formed when
Concept Introduction:
Pyridine is a heterocyclic compound which contains nitrogen atom. Pyridine nitrogen atom contain a lone pair. The lone pair of pyridine is not involved in the resonance with the aromatic ring. Hydrogen peroxide is a strong oxidizing agent. It will oxidize the nitrogen atom of pyridine to form
Answer to Problem 26.27AP
The product formed when
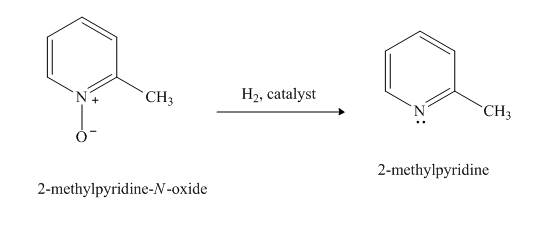
Explanation of Solution
The compound

Figure 8
The product formed when
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry
- Organic Functional Groups Predicting the reactants or products of acetal hydrolysis termine the structures of the missing organic molecules in the following reaction: H* H* + H₂O Y ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure Explanation Check @2 W Click and drag to start drawing a structure. #4 # 3 LU E % 67 olo 5 66 R T Y & 7 AcGraw Hill LLC. All Rights R Xarrow_forward8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enaminearrow_forwardDraw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forward
- Complete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forwardPredict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forward
- Predict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

